0000819050FYFALSE2023P3Y0000008190502023-01-012023-12-3100008190502023-06-30iso4217:USD00008190502024-03-15xbrli:shares00008190502023-12-3100008190502022-12-31iso4217:USDxbrli:shares0000819050us-gaap:ProductAndServiceOtherMember2023-01-012023-12-310000819050us-gaap:ProductAndServiceOtherMember2022-01-012022-12-310000819050us-gaap:RoyaltyMember2023-01-012023-12-310000819050us-gaap:RoyaltyMember2022-01-012022-12-3100008190502022-01-012022-12-310000819050us-gaap:SeriesAPreferredStockMember2021-12-310000819050us-gaap:CommonStockMember2021-12-310000819050us-gaap:AdditionalPaidInCapitalMember2021-12-310000819050us-gaap:RetainedEarningsMember2021-12-3100008190502021-12-310000819050us-gaap:SeriesAPreferredStockMember2022-01-012022-12-310000819050us-gaap:CommonStockMember2022-01-012022-12-310000819050us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310000819050us-gaap:RetainedEarningsMember2022-01-012022-12-310000819050us-gaap:SeriesAPreferredStockMember2022-12-310000819050us-gaap:CommonStockMember2022-12-310000819050us-gaap:AdditionalPaidInCapitalMember2022-12-310000819050us-gaap:RetainedEarningsMember2022-12-310000819050us-gaap:CommonStockMember2023-01-012023-12-310000819050us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310000819050us-gaap:RetainedEarningsMember2023-01-012023-12-310000819050us-gaap:SeriesAPreferredStockMember2023-12-310000819050us-gaap:CommonStockMember2023-12-310000819050us-gaap:AdditionalPaidInCapitalMember2023-12-310000819050us-gaap:RetainedEarningsMember2023-12-31frtx:segment0000819050srt:MinimumMember2023-12-310000819050srt:MaximumMember2023-12-310000819050us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2023-12-310000819050us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2022-12-310000819050us-gaap:WarrantMember2023-01-012023-12-310000819050us-gaap:WarrantMember2022-01-012022-12-310000819050us-gaap:EmployeeStockOptionMember2023-01-012023-12-310000819050us-gaap:EmployeeStockOptionMember2022-01-012022-12-3100008190502022-05-2500008190502022-07-012022-07-310000819050frtx:VoronoiIncMember2021-08-272021-08-2700008190502021-08-272021-08-27xbrli:pure0000819050frtx:CarnaBiosciencesIncMember2022-02-020000819050srt:MaximumMemberfrtx:KakenMemberfrtx:MilestonePaymentsMember2022-05-030000819050srt:MaximumMemberus-gaap:RoyaltyMemberfrtx:KakenMember2022-05-030000819050us-gaap:RoyaltyMemberus-gaap:CollaborativeArrangementMemberfrtx:BotanixMember2022-01-012022-12-310000819050frtx:BotanixMember2023-07-212023-07-210000819050frtx:BotanixMemberfrtx:BodorLaboratoriesInc.Member2023-07-212023-07-210000819050frtx:BotanixMember2022-05-030000819050frtx:MilestonePaymentsMemberfrtx:BotanixMember2022-05-030000819050srt:MaximumMemberfrtx:MilestonePaymentsMemberfrtx:PaymentCriteriaOneMemberfrtx:BotanixMember2022-05-030000819050frtx:MilestonePaymentsMemberfrtx:PaymentCriteriaTwoMemberfrtx:BotanixMember2022-05-030000819050frtx:PaymentCriteriaThreeMemberfrtx:BotanixMember2022-05-030000819050frtx:ConsultingServicesMemberfrtx:BotanixMember2022-05-032022-05-030000819050frtx:BotanixMember2022-05-032022-05-030000819050frtx:BuyoutOfPostClosingObligationsMemberfrtx:BotanixMember2023-01-012023-12-310000819050frtx:BuyoutOfPostClosingObligationsMemberfrtx:BotanixMember2022-01-012022-12-310000819050frtx:BotanixMemberfrtx:SublicenseIncomeMember2023-01-012023-12-310000819050frtx:BotanixMemberfrtx:SublicenseIncomeMember2022-01-012022-12-310000819050frtx:ConsultingServicesMemberfrtx:BotanixMember2023-01-012023-12-310000819050frtx:ConsultingServicesMemberfrtx:BotanixMember2022-01-012022-12-310000819050frtx:UpfrontConsiderationReceivedFromBotanixMemberfrtx:BotanixMember2023-01-012023-12-310000819050frtx:UpfrontConsiderationReceivedFromBotanixMemberfrtx:BotanixMember2022-01-012022-12-310000819050frtx:MilestonePaymentReceivedUponAcceptanceByFDAOfNDAFilingMemberfrtx:BotanixMember2023-01-012023-12-310000819050frtx:MilestonePaymentReceivedUponAcceptanceByFDAOfNDAFilingMemberfrtx:BotanixMember2022-01-012022-12-310000819050frtx:ReimbursedDevelopmentExpendituresUnderTheAssetPurchaseAgreementMemberfrtx:BotanixMember2023-01-012023-12-310000819050frtx:ReimbursedDevelopmentExpendituresUnderTheAssetPurchaseAgreementMemberfrtx:BotanixMember2022-01-012022-12-310000819050frtx:BotanixMember2023-01-012023-12-310000819050frtx:BotanixMember2022-01-012022-12-310000819050frtx:BotanixMember2022-12-310000819050frtx:BotanixMember2023-12-310000819050frtx:BotanixMember2021-12-310000819050frtx:BodorLaboratoriesInc.Member2022-05-032022-05-0300008190502022-11-102022-11-100000819050frtx:BodorLaboratoriesInc.Member2022-11-102022-11-100000819050frtx:BodorLaboratoriesInc.Member2023-01-012023-12-310000819050frtx:BodorLaboratoriesInc.Member2022-01-012022-12-3100008190502023-05-04frtx:vote0000819050us-gaap:WarrantMember2023-12-310000819050us-gaap:CommonStockMemberfrtx:October2020OfferingMember2020-10-012020-10-310000819050frtx:CommonStockWarrantsMemberfrtx:October2020OfferingMember2020-10-310000819050frtx:October2020OfferingMember2020-10-310000819050frtx:CommonStockWarrantsMember2020-10-3100008190502020-10-012020-10-310000819050frtx:CommonStockPublicOfferingMember2020-06-300000819050frtx:PreFundedWarrantMemberfrtx:CommonStockPublicOfferingMember2020-06-300000819050frtx:CommonStockPublicOfferingMemberfrtx:AccompanyingCommonWarrantMember2020-06-300000819050frtx:CommonStockPublicOfferingMember2020-06-012020-06-300000819050frtx:A2021AtMarketIssuanceSalesAgreementMember2021-03-310000819050frtx:A2021AtMarketIssuanceSalesAgreementMember2023-01-012023-12-310000819050frtx:A2021AtMarketIssuanceSalesAgreementMember2022-01-012022-12-310000819050frtx:A2021AtMarketIssuanceSalesAgreementMember2023-12-310000819050frtx:A2020AtMarketIssuanceSalesAgreementMember2020-04-300000819050frtx:A2020AtMarketIssuanceSalesAgreementMember2022-01-012022-12-310000819050frtx:A2020AtMarketIssuanceSalesAgreementMember2023-01-012023-12-310000819050frtx:A2020AtMarketIssuanceSalesAgreementScenario2Member2023-12-310000819050frtx:PrivatePlacementOfferingsMember2020-02-290000819050frtx:PrivatePlacementOfferingsMemberus-gaap:SeriesAMember2020-02-290000819050frtx:PrivatePlacementOfferingsMemberus-gaap:SeriesBMember2020-02-290000819050frtx:PurchaseAgreementMemberfrtx:LincolnParkMember2020-02-2900008190502022-05-252022-05-250000819050us-gaap:EmployeeStockOptionMember2023-01-012023-12-310000819050us-gaap:EmployeeStockOptionMember2022-01-012022-12-310000819050us-gaap:EmployeeStockOptionMembersrt:WeightedAverageMember2022-01-012022-12-310000819050us-gaap:EmployeeStockOptionMember2022-12-310000819050us-gaap:EmployeeStockOptionMember2023-12-310000819050us-gaap:RestrictedStockUnitsRSUMember2022-12-310000819050us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310000819050us-gaap:RestrictedStockUnitsRSUMember2023-12-310000819050us-gaap:RestrictedStockUnitsRSUMember2023-12-282023-12-280000819050us-gaap:SubsequentEventMemberus-gaap:RestrictedStockUnitsRSUMember2024-01-012024-01-310000819050us-gaap:EmployeeStockMemberfrtx:EmployeeStockPurchasePlanMember2023-01-012023-12-310000819050us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-12-310000819050us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-12-310000819050us-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-12-310000819050us-gaap:GeneralAndAdministrativeExpenseMember2022-01-012022-12-310000819050us-gaap:EmployeeStockMember2023-01-012023-12-310000819050us-gaap:DomesticCountryMember2023-12-310000819050us-gaap:DomesticCountryMember2022-12-310000819050frtx:NOLsGeneratedAfter2017Memberus-gaap:DomesticCountryMember2023-12-310000819050frtx:NOLsGeneratedBefore2018Memberus-gaap:DomesticCountryMember2023-12-310000819050frtx:FederalResearchAndDevelopmentCreditsAndOrphanDrugCreditMemberus-gaap:DomesticCountryMember2023-12-310000819050frtx:FederalResearchAndDevelopmentCreditsAndOrphanDrugCreditMemberus-gaap:DomesticCountryMember2022-12-310000819050us-gaap:StateAndLocalJurisdictionMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMember2022-12-310000819050frtx:TaxYear2024Memberfrtx:Sections382And383Memberus-gaap:DomesticCountryMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMemberfrtx:TaxYear2024Memberfrtx:Sections382And383Member2023-12-310000819050frtx:FederalResearchAndDevelopmentMemberfrtx:TaxYear2024Member2023-12-310000819050frtx:TaxYear2024Memberfrtx:FederalOrphanDrugMember2023-12-310000819050frtx:TaxYear2024Memberfrtx:StateResearchAndDevelopmentMember2023-12-310000819050frtx:TaxYear2025Memberfrtx:Sections382And383Memberus-gaap:DomesticCountryMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMemberfrtx:TaxYear2025Memberfrtx:Sections382And383Member2023-12-310000819050frtx:FederalResearchAndDevelopmentMemberfrtx:TaxYear2025Member2023-12-310000819050frtx:TaxYear2025Memberfrtx:FederalOrphanDrugMember2023-12-310000819050frtx:TaxYear2025Memberfrtx:StateResearchAndDevelopmentMember2023-12-310000819050frtx:TaxYear2026Memberfrtx:Sections382And383Memberus-gaap:DomesticCountryMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMemberfrtx:TaxYear2026Memberfrtx:Sections382And383Member2023-12-310000819050frtx:FederalResearchAndDevelopmentMemberfrtx:TaxYear2026Member2023-12-310000819050frtx:TaxYear2026Memberfrtx:FederalOrphanDrugMember2023-12-310000819050frtx:TaxYear2026Memberfrtx:StateResearchAndDevelopmentMember2023-12-310000819050frtx:TaxYear2027AndThereafterMemberfrtx:Sections382And383Memberus-gaap:DomesticCountryMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMemberfrtx:TaxYear2027AndThereafterMemberfrtx:Sections382And383Member2023-12-310000819050frtx:FederalResearchAndDevelopmentMemberfrtx:TaxYear2027AndThereafterMember2023-12-310000819050frtx:TaxYear2027AndThereafterMemberfrtx:FederalOrphanDrugMember2023-12-310000819050frtx:TaxYear2027AndThereafterMemberfrtx:StateResearchAndDevelopmentMember2023-12-310000819050frtx:IndefiniteMemberfrtx:Sections382And383Memberus-gaap:DomesticCountryMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMemberfrtx:IndefiniteMemberfrtx:Sections382And383Member2023-12-310000819050frtx:FederalResearchAndDevelopmentMemberfrtx:IndefiniteMember2023-12-310000819050frtx:IndefiniteMemberfrtx:FederalOrphanDrugMember2023-12-310000819050frtx:IndefiniteMemberfrtx:StateResearchAndDevelopmentMember2023-12-310000819050frtx:Sections382And383Memberus-gaap:DomesticCountryMember2023-12-310000819050us-gaap:StateAndLocalJurisdictionMemberfrtx:Sections382And383Member2023-12-310000819050frtx:FederalResearchAndDevelopmentMember2023-12-310000819050frtx:FederalOrphanDrugMember2023-12-310000819050frtx:StateResearchAndDevelopmentMember2023-12-310000819050us-gaap:DomesticCountryMember2023-01-012023-12-310000819050us-gaap:ForeignCountryMember2023-01-012023-12-3100008190502023-10-012023-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(mark one)
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2023
or
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 000-21088
FRESH TRACKS THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | | | | | | | | | | | | | |
| Delaware | 93-0948554 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| | | | | | | |
| 2000 Central Avenue, | Suite 100, | Boulder, | CO | | 80301 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (720) 505-4755
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading symbol(s) | Name of each exchange on which registered |
N/A | N/A | N/A |
Securities registered pursuant to section 12(g) of the Act: Common stock, $0.01 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |
| | Emerging growth company | ☐ | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant, based upon the closing sale price of the registrant’s common stock on June 30, 2023, as reported on The Nasdaq Capital Market, was $4.1 million. Shares of common stock held by each executive officer and director and by each person who owns 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 15, 2024, there were 5,973,306 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
FRESH TRACKS THERAPEUTICS, INC.
INDEX
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (“Annual Report”) contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements contained in this Annual Report other than statements of historical fact, including statements relating to future financial, business, conditions, plans, prospects, impacts, shifts, trends, progress, or strategies and other such matters, including without limitation, our proposed liquidation and dissolution (the “Dissolution”) pursuant to our proposed plan of liquidation and dissolution (the “Plan of Dissolution”), the timing of filing of the Certificate of Dissolution, the Company’s intent to continue to seek approval to dissolve and the results of such action, the amount, number, and timing of liquidating distributions, if any, to our stockholders, the amount of reserves, and similar statements, are forward-looking statements. The words “may,” “could,” “should,” “might,” “delist,” “suspend,” “appeal,” “request,” “stay,” “notify,” “cancel,” “expeditiously,” “quickly,” “approve,” “show,” “maximize,” “advise,” “continue,” “additional,” “range,” “announce,” “anticipate,” “explore,” “reflect,” “believe,” “sufficient,” “transform,” “estimate,” “expect,” “intend,” “plan,” “file,” “make,” “timely,” “promptly,” “attempt,” “distribute,” “discontinue,” “dissolve,” “dissolution,” “wind down,” “best interests,” “predict,” “potential,” “will,” evaluate,” “aim,” “help,” “progress,” “meet,” “support,” “look forward,” “develop,” “strengthen,” “promise,” “successful,” “positive,” “provide,” “commit,” “opportunity,” “disrupt,” “reduce,” “restore,” “demonstrate,” “suggest,” “target,” “shift,” “inhibit,” and similar expressions and their variants, are intended to identify forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors. Unless otherwise mentioned or unless the context requires otherwise, all references in this Annual Report to “Fresh Tracks,” “Brickell Subsidiary,” “Company,” “we,” “us,” and “our,” or similar references, refer to Fresh Tracks Therapeutics, Inc. and its consolidated subsidiaries.
We based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our proposed Dissolution and Plan of Dissolution, status and return of product licenses and management, wind down of Company operations and assets, financial condition, results of operations, business operations and objectives, employees, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in Part I, Item 1A, “Risk Factors” in this Annual Report and under a similar heading in any other periodic or current report we may file with the U.S. Securities and Exchange Commission (the “SEC”) in the future. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge quickly and from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business and operations or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions, the future events and trends discussed in this Annual Report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement. You should read carefully the factors described in Part I, Item 1A, “Risk Factors” in this Annual Report and under a similar heading in any other periodic or current report we may file with the SEC to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. You are advised to consult any further disclosures we make on related subjects in our future public filings and on our website.
RISK FACTORS SUMMARY
Our business, financial condition, and operating results may be affected by a number of factors, whether currently known or unknown. Any one or more of such factors could directly or indirectly cause our actual results of operations and financial condition to vary materially from past or anticipated future results of operations and financial condition. Any of these factors, in whole or in part, let alone combined with any of the others, could materially and adversely affect our business, financial condition, results of operations, and stock price. We have provided a summary of some of these risks below, with a more detailed explanation of those and other risks applicable to the Company in Part I, Item 1A. “Risk Factors” in this Annual Report. On September 19, 2023, we announced the Plan of Dissolution and our intent to discontinue all clinical and preclinical development programs and reduce our workforce. In connection with the Plan of Dissolution, effective October 2, 2023, we discontinued all clinical and preclinical development programs and terminated most of our employees, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company. We held special meetings of stockholders on November 16, 2023, November 30, 2023, December 15, 2023, December 27, 2023, and February 15, 2024 (the “Special Meetings”) to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of our common stock entitled to vote at the Special Meetings, and as a result, we intend to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time. The following risks are related to the Dissolution:
•We cannot assure you as to the timing, amount, or number of distributions, if any, to be made to our stockholders.
•The Board of Directors (“Board”) may determine not to proceed with the Dissolution, or the Company may not obtain the necessary approval to effect the Dissolution.
•Our stockholders may be liable to third parties for part or all of the amount received from us in our liquidating distributions if cash reserves are inadequate.
•Our stockholders of record will not be able to buy or sell shares of our common stock after we close our stock transfer books at the effective time of the Dissolution (the “Effective Time”).
•We plan to initiate steps soon to exit from certain reporting requirements under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which may substantially reduce publicly available information about us. If the exit process is protracted, we will continue to bear the expense of being a public reporting company despite having no source of revenue.
•The loss of key personnel could adversely affect our ability to efficiently dissolve, liquidate, and wind down.
PART I.
ITEM 1. BUSINESS
On September 19, 2023, we announced the Plan of Dissolution and our intent to discontinue all clinical and preclinical development programs and reduce our workforce. Historically, we were a clinical-stage pharmaceutical company striving to transform patient lives through the development of innovative and differentiated prescription therapeutics. Our pipeline aimed to disrupt existing treatment paradigms and featured several new chemical entities that inhibit novel targets with first-in-class potential for autoimmune, inflammatory, and other debilitating diseases.
Our Board and executive management team conducted a comprehensive process to explore and evaluate strategic alternatives with the goal of maximizing stockholder value. Potential alternatives that were under evaluation included, but were not limited to, a financing, a merger or reverse merger, the sale of all or part of the Company, licensing of assets, a business combination, and/or other strategic transactions or series of related transactions involving the Company.
On September 18, 2023, after conducting an extensive, months-long potential strategic alternatives process, including four unsuccessful attempts to find a merger or reverse merger partner due to the potential acquirer’s inability to secure its own necessary financing and/or inability to offer adequate value to consummate the transaction, and combined with the unsuccessful outreach to approximately 125 other possible counterparties and investors who operate or invest in both life sciences and other industry sectors, our Board unanimously approved the Dissolution and the Plan of Dissolution, subject to the approval of our stockholders. In connection with the Plan of Dissolution, effective October 2, 2023, we discontinued all clinical and preclinical development programs and terminated most of our employees, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company.
We held Special Meetings on November 16, 2023, November 30, 2023, December 15, 2023, December 27, 2023, and February 15, 2024 to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of our common stock entitled to vote at the Special Meetings, and as a result, we intend to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time.
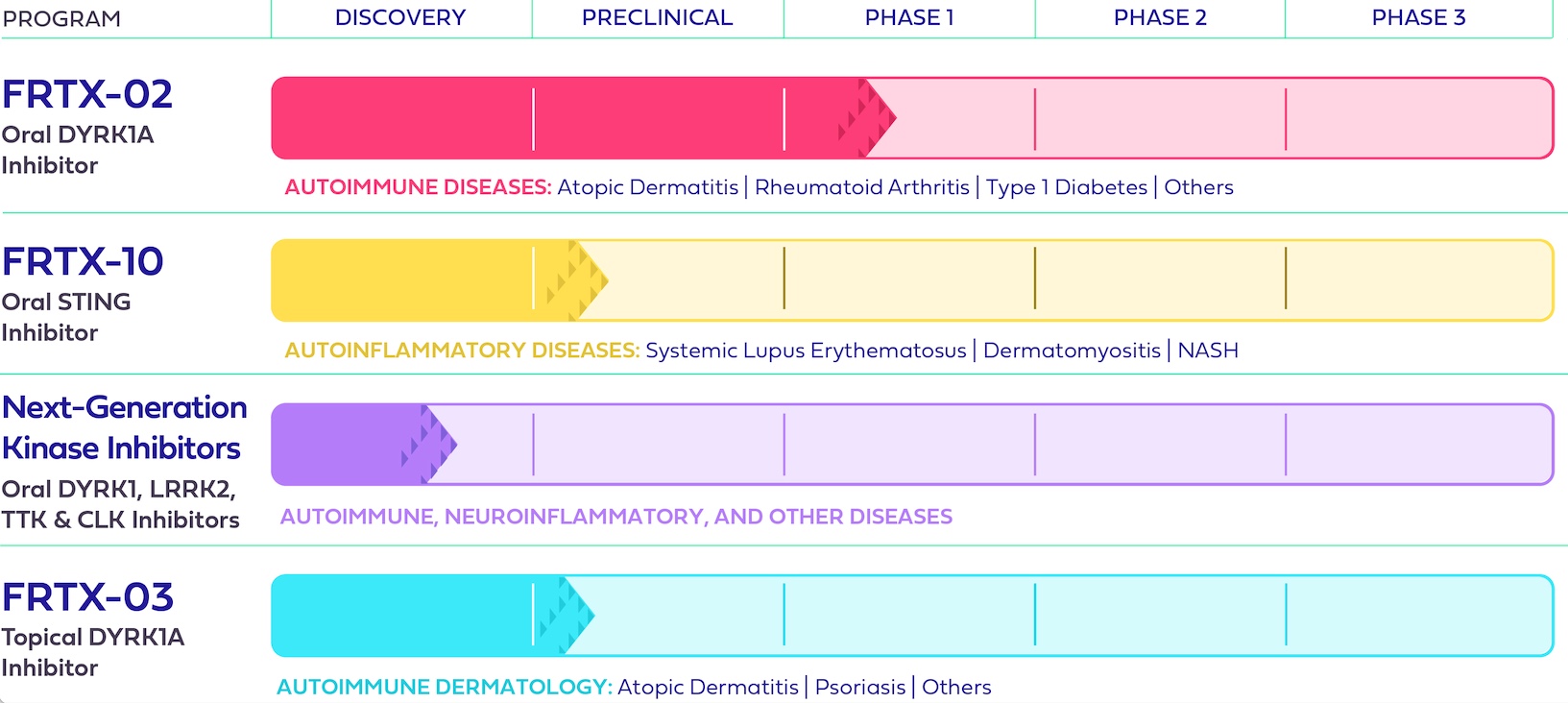
Research and Development Assets
The following image summarizes our current or previous research and development assets, corresponding stage of development, and potential therapeutic areas for each program:
Research & Development Programs
FRTX-02: A Potential First-in-Class Oral DYRK1A Inhibitor for the Treatment of Autoimmune and Inflammatory Diseases
FRTX-02 is a novel, potent, highly selective, and orally bioavailable potential first-in-class, small molecule DYRK1A inhibitor that aims to restore immune balance in patients whose immune systems have become dysregulated. FRTX-02 was our lead development-stage program and has demonstrated promising results in various preclinical and clinical models, including potentially for atopic dermatitis (“AD”) and rheumatoid arthritis.
FRTX-10: A covalent Stimulator of Interferon Genes (STING) inhibitor for the Potential Treatment of Autoimmune, Inflammatory, and Rare Genetic Diseases
FRTX-10 is an early-stage Stimulator of Interferon Genes (“STING”) inhibitor candidate and a novel, potent, and orally bioavailable covalent STING inhibitor that specifically targets the palmitoylation site of STING. STING is a well-known mediator of innate immune responses. Excessive signaling through STING is linked to numerous high unmet-need diseases, ranging from autoimmune disorders, such as systemic lupus erythematosus, to interferonopathies, which are a set of rare genetic conditions characterized by interferon overproduction and could have orphan drug potential. Effective March 1, 2024, the license to develop FRTX-10 was terminated by mutual agreement.
Next-Generation Kinase Inhibitors: A Cutting-Edge Platform with the Potential to Produce Treatments for Autoimmune, Inflammatory, and Other Debilitating Diseases
We have global rights to a cutting-edge platform of next-generation kinase inhibitors. This library of new chemical entities includes next-generation DYRK1 inhibitors, as well as other molecules that specifically inhibit Leucine-Rich Repeat Kinase 2 (“LRRK2”), CDC2-like kinase (“CLK”), and TTK protein kinase (“TTK”), also known as Monopolar spindle 1 (Mps1) kinases. A number of these drug candidates have the potential to penetrate the blood-brain barrier, presenting an opportunity to address neuroinflammatory conditions of high
unmet need, such as Down Syndrome, Alzheimer’s Disease, and Parkinson’s Disease, while other peripherally acting novel LRRK2, TTK, and CLK kinase inhibitors could be developed in additional therapeutic areas within autoimmunity, inflammation, and oncology.
Intellectual Property
Patents extend for varying periods according to the date of patent filing or grant, applicable laws allowing for patent term extension, and the legal term of patents in various countries where patent protection is obtained. The actual protection afforded by a patent can vary from country to country and depends on the type of patent, the scope of its coverage, and the availability of legal remedies in the country.
Under the terms of the Voronoi License Agreement, the Company is responsible for the development and commercialization activities, including the first right to prosecute and maintain patents, related to all the licensed compounds. FRTX-02 is covered by a composition of matter patent issued in the U.S., Japan, China, and other key countries through at least 2038, subject to patent term extensions and adjustments that may be available depending on how this early-stage asset is developed, as well as a pending Patent Cooperation Treaty (“PCT”) application, and other foreign and U.S. applications for FRTX-02, as of the date of this Annual Report. Compounds from the next-generation kinase inhibitor platform are covered by U.S. and foreign composition of matter patent applications, as well as other applications, that are currently pending in global prosecution and being managed directly by our licensor. The Company continues to assess prosecution deadlines for the licensed patents and has so far elected to transfer the prosecution, maintenance, and costs of managing such patents directly to our licensor.
We protect our proprietary information by requiring any of our directors, officers, employees, consultants, contractors, and other advisors to execute nondisclosure and assignment of invention agreements upon commencement of their respective employment or engagement. Agreements with our employees also prevent them from bringing the proprietary rights of third parties to our company without adequate permission to do so. In addition, we require confidentiality or service agreements from third parties that receive our confidential information or materials.
Strategic, Licensing, and Other Arrangements
License and Development Agreement with Voronoi
In August 2021, we entered into a License and Development Agreement (the “Voronoi License Agreement”) with Voronoi Inc. (“Voronoi”), pursuant to which we acquired exclusive, worldwide rights to research, develop, and commercialize FRTX-02 and other next-generation kinase inhibitors.
With respect to FRTX-02, the Voronoi License Agreement provides that we will make payments to Voronoi of up to $211.0 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. With respect to the compounds arising from the next-generation kinase inhibitor platform, we will make payments to Voronoi of up to $107.5 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. Further, the Voronoi License Agreement provides that we will pay Voronoi tiered royalty payments ranging from low-single digits up to 10% of net sales of products arising from the DYRK1A inhibitor programs and next-generation kinase inhibitor platform. All of the contingent payments and royalties are payable in cash in U.S. Dollars, except for $1.0 million of the development and regulatory milestone payments, which amount is payable in equivalent shares of our common stock. Under the terms of the Voronoi License Agreement, we are responsible for, and bear the future costs of, all development and commercialization activities, including the first right to prosecute and maintain patents, related to all the licensed compounds. As of December 31, 2023 and through the date of this Annual Report, we have not yet made any payments or recorded any liabilities related to the specified
development, regulatory, and commercial milestones or royalties on net sales pursuant to the Voronoi License Agreement.
The Voronoi License Agreement also provides that upon termination of the Voronoi License Agreement, Voronoi will be entitled to receive a non-exclusive license to any information and know-how independently developed by the Company for FRTX-02 and other licensed assets in consideration for payment(s) at an arms’-length royalty rate on net sales that must be negotiated in good faith between the parties.
Exclusive License and Development Agreement with Carna
In February 2022, we entered into an Exclusive License Agreement (the “Carna License Agreement”) with Carna Biosciences, Inc. (“Carna”), pursuant to which we acquired exclusive, worldwide rights to research, develop, and commercialize Carna’s portfolio of novel STING inhibitors. In accordance with the terms of the Carna License Agreement, in exchange for the licensed rights, we made a one-time cash payment of $2.0 million.
The Carna License Agreement provided that we would make success-based payments to Carna of up to $258.0 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. Further, the Carna License Agreement provided that we would pay Carna tiered royalty payments ranging from mid-single digits up to 10% of net sales. Under the terms of the Carna License Agreement, we were responsible for all development and commercialization activities, including patenting, related to all the licensed compounds. As of December 31, 2023 and through the date of this Annual Report, we have not made any payments or recorded any liabilities related to the specified development, regulatory, and commercial milestones or royalties on net sales pursuant to the Carna License Agreement.
Effective March 1, 2024, the Carna License Agreement was terminated by mutual agreement.
Agreements with Botanix
Asset Purchase Agreement with Botanix
On May 3, 2022 (the “Effective Date”), we and Brickell Subsidiary, Inc. (“Brickell Subsidiary”) entered into an asset purchase agreement with Botanix SB, Inc. and Botanix Pharmaceuticals Limited (“Botanix”) (the “Asset Purchase Agreement”), pursuant to which Botanix acquired and assumed control of all rights, title, and interests to assets primarily related to the proprietary compound sofpironium bromide that were owned and/or licensed by us or Brickell Subsidiary (the “Assets”). Prior to the sale of the Assets, we had previously entered into a License Agreement with Bodor Laboratories, Inc. (“Bodor”), dated December 15, 2012 (last amended in February 2020) that provided us with a worldwide exclusive license to develop, manufacture, market, sell, and sublicense products containing sofpironium bromide through which the Assets were developed (the “Amended and Restated License Agreement”). As a result of the Asset Purchase Agreement, Botanix became responsible for all further research, development, and commercialization of sofpironium bromide globally and replaced us as the exclusive licensee under the Amended and Restated License Agreement.
In accordance with the sublicense rights provided to us under the Amended and Restated License Agreement, we also had previously entered into a License, Development, and Commercialization Agreement with Kaken Pharmaceutical Co., Ltd. (“Kaken”), dated as of March 31, 2015 (as amended in May 2018, the “Kaken Agreement”), under which we granted to Kaken an exclusive right to develop, manufacture, and commercialize the sofpironium bromide compound in Japan and certain other Asian countries (the “Territory”). In exchange for the sublicense, we were entitled to receive aggregate payments of up to $10.0 million upon the achievement of specified development milestones, which were earned and received in 2017 and 2018, and up to $19.0 million upon the achievement of sales-based milestones, as well as tiered royalties based on a percentage of net sales of licensed products in the Territory. In September 2020, Kaken received regulatory approval in
Japan to manufacture and market sofpironium bromide gel, 5% (“ECCLOCK®”) for the treatment of primary axillary hyperhidrosis, and as a result, we began recognizing royalty revenue earned on a percentage of net sales of ECCLOCK in Japan. Pursuant to the Asset Purchase Agreement, the Kaken Agreement was assigned to Botanix, which replaced us as the exclusive sub-licensor to Kaken.
We determined that the development of and ultimate sale and assignment of rights to the Assets is an output of our ordinary activities and Botanix is a customer as it relates to the sale of the Assets and related activities.
On July 21, 2023, we and Brickell Subsidiary entered into Amendment No. 1 to the Asset Purchase Agreement (the “Asset Purchase Agreement Amendment”) with Botanix. The Asset Purchase Agreement Amendment provided that, in lieu of any remaining amounts potentially payable by Botanix to us pursuant to the Asset Purchase Agreement (collectively, the “Post-Closing Payment Obligations”), Botanix would pay $6.6 million to us and $1.7 million on behalf of us to Bodor. The payments from Botanix to the Company and Bodor were made on July 26, 2023. The Asset Purchase Agreement Amendment also provided that upon payment of the amounts by Botanix thereunder, all Post-Closing Payment Obligations under the Asset Purchase Agreement were terminated and of no further force or effect.
In accordance with the terms of the Asset Purchase Agreement, in exchange for the Assets, we (i) received an upfront payment at closing in the amount of $3.0 million, (ii) were reimbursed for certain recent development expenditures in advancement of the Assets, (iii) received a milestone payment of $2.0 million upon the acceptance by the U.S. Food and Drug Administration (“FDA”) in December 2022 of the filing of a new drug application (“NDA”) for sofpironium bromide gel, 15%, and (iv) would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, a contingent milestone payment of $4.0 million if marketing approval in the U.S. for sofpironium bromide gel, 15%, had been received on or before September 30, 2023, or $2.5 million if such marketing approval had been received after September 30, 2023 but on or before February 17, 2024. Botanix submitted an NDA for sofpironium bromide gel, 15%, to the FDA in September 2022, which was accepted for filing by the FDA in December 2022. Under the Asset Purchase Agreement, we also would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, additional success-based regulatory and sales milestone payments of up to $168.0 million. Further, we would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, tiered earnout payments ranging from high-single digits to mid-teen digits on net sales of sofpironium bromide gel (the “Earnout Payments”).
The Asset Purchase Agreement also provided that Botanix would pay us a portion of the sales-based milestone payments and royalties that Botanix received from Kaken under the assigned Kaken Agreement (together, the “Sublicense Income”). Sublicense Income represented our estimate of payments that would be earned by us in the applicable period from sales-based milestone payments and royalties Botanix would receive from Kaken to the extent it was probable that a significant reversal in the amount of cumulative revenue recognized would not occur. Royalties vary based on net sales that are impacted by a wide variety of market and other factors. We recorded a contract asset equal to the amount of revenue recognized related to the Sublicense Income, less the amount of payments received from or due by Botanix in relation to the Sublicense Income.
All other consideration due under the Asset Purchase Agreement was contingent upon certain regulatory approvals and future sales subsequent to such regulatory approvals, or was based upon future sales that we determined were not yet probable due to such revenues being highly susceptible to factors outside of our influence and uncertainty about the amount of such consideration that would not be resolved for an extended period of time. Therefore, we determined that such variable consideration amounts were fully constrained up through the date of the Asset Purchase Agreement Amendment, and, as such, did not recognize such amounts as contract revenue.
Transition Services Agreement with Botanix
In connection with the sale of the Assets, on the Effective Date, we and Botanix entered into a transition services agreement (the “TSA”) whereby we provide consulting services as an independent contractor to Botanix in support of and through filing and potential approval of the U.S. NDA for sofpironium bromide gel, 15%. In accordance with the terms of the TSA, in exchange for providing these services, (i) prior to the acceptance of the filing by the FDA of such NDA in December 2022, we received from Botanix a fixed monthly amount of $71 thousand, and (ii) after the acceptance of the filing in December 2022, we receive from Botanix, a variable amount based upon actual hours worked, in each case plus related fees and expenses of our advisors (plus a 5% administrative fee) and our out-of-pocket expenses. As of the date of this Annual Report, we do not expect to provide any further services or receive any additional fees related to the TSA.
Contract Revenue under the Botanix Agreements
During the year ended December 31, 2023, we recorded contract revenue of $8.0 million. For additional information regarding contract revenue described above, see Note 3. “Strategic Agreements” of the notes to our consolidated financial statements included in this Annual Report.
Agreements with Bodor
In connection with the sale of the Assets, on the Effective Date, we, Brickell Subsidiary, and Bodor entered into an agreement (the “Rights Agreement”) to clarify that we and Brickell Subsidiary have the power and authority under the Amended and Restated License Agreement to enter into the Asset Purchase Agreement and the TSA, and that Botanix would assume the Amended and Restated License Agreement pursuant to the Asset Purchase Agreement. The Rights Agreement included a general release of claims and no admission of liability between the parties. Pursuant to such Rights Agreement, as subsequently amended on November 10, 2022, we agreed to pay Bodor (i) 20% of the amount of each payment due to us from Botanix for upfront and milestone payments, subject to deductions, credits, or offsets applied under the Asset Purchase Agreement, as well as (ii) certain tiered payments, set as a percentage ranging from mid-single digits to mid-teen digits, of the amount of each of the applicable Earnout Payments due to us from Botanix after deductions, credits, or offsets applied under the Asset Purchase Agreement.
Pursuant to the terms of the Asset Purchase Agreement, we retained our obligation under the Amended and Restated License Agreement to issue $1.0 million in shares of our common stock to Bodor upon the FDA’s acceptance of an NDA filing for sofpironium bromide gel, 15%. On November 10, 2022, we entered into an Acknowledgment and Agreement Related to Asset Purchase Agreement and Amended and Restated License Agreement (the “Acknowledgment”) with Brickell Subsidiary, Botanix, and Bodor. Pursuant to the Acknowledgment, we paid $1.0 million in cash to Bodor in full satisfaction of our obligation to issue shares upon the FDA’s acceptance of the NDA. We determined to prepay this obligation in cash in order to avoid the substantial dilution to our stockholders that would have resulted if we had issued the shares of our common stock originally provided for in the Amended and Restated License Agreement.
In connection with the Asset Purchase Agreement Amendment, on July 21, 2023, we, Brickell Subsidiary, and Bodor entered into a Second Amendment to Rights Agreement (the “RA Amendment”). The RA Amendment provides that in exchange for the one-time payment of $1.7 million by Botanix on behalf of us to Bodor, we shall have no further payment obligations to Bodor under or in connection with the Rights Agreement or the Amended and Restated License Agreement. Except as explicitly amended by the RA Amendment, the Rights Agreement remains in full force and effect.
During the year ended December 31, 2023, we incurred $1.7 million of general and administrative expenses associated with payments due to Bodor. For additional information regarding obligations due to Bodor
described above, see Note 3. “Strategic Agreements” of the notes to our consolidated financial statements included in this Annual Report.
Manufacturing and Supply
Because we discontinued all clinical and preclinical development programs in October 2023, we currently do not have any contracts with third parties for the manufacture of drug substances and drug products for use in nonclinical and clinical studies.
Government Regulation
Although our operations are currently focused on winding down our operations in connection with our anticipated Dissolution, we remain subject to numerous federal, state and local laws and regulations, including securities, tax, anti-bribery and privacy laws and regulations.
Employees
As of December 31, 2023, we had four full-time employees. In October 2023, we discontinued all clinical and preclinical development programs and terminated most of our employees, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company.
Corporate History
Vical Incorporated (“Vical”) was incorporated in Delaware in 1987. On August 31, 2019, the Delaware corporation formerly known as “Vical Incorporated” completed a reverse merger transaction in accordance with the terms and conditions of the Agreement and Plan of Merger and Reorganization, dated June 2, 2019, as further amended on August 20, 2019 and August 30, 2019, by and among Vical, Brickell Biotech, Inc. (“Private Brickell”) and Victory Subsidiary, Inc. (“Merger Sub”), pursuant to which Merger Sub merged with and into Private Brickell, with Private Brickell surviving the merger as a wholly-owned subsidiary of Vical (the “Merger”). Additionally, on August 31, 2019, immediately after the completion of the Merger, the Company changed its name from “Vical Incorporated” to “Brickell Biotech, Inc.” On September 7, 2022, Brickell Biotech, Inc.’s name was changed to Fresh Tracks Therapeutics, Inc.
Corporate Information
Our corporate headquarters are in Boulder, Colorado, where we maintain our corporate offices at 2000 Central Avenue, Suite 100, Boulder, CO 80301 under a virtual office lease. We lease our corporate office premises under a monthly rental agreement at a nominal cost. We consider our current office space adequate for our current operations.
This Annual Report contains references to our trademarks and trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this Annual Report, including logos, artwork, and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
ITEM 1A. RISK FACTORS
Our business, financial condition, and operating results may be affected by a number of factors, whether currently known or unknown, including but not limited to those described below. Any one or more of such factors could directly or indirectly cause our actual results of operations and financial condition to vary
materially from past or anticipated future results of operations and financial condition. Any of these factors, in whole or in part, alone or combined with any of the other factors, could materially and adversely affect our business, financial condition, results of operations, and stock price. The following information should be read in conjunction with Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and related notes in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report.
Risks Related to the Dissolution
We cannot predict the timing of the distributions, if any, to stockholders.
We held Special Meetings on November 16, 2023, November 30, 2023, December 15, 2023, December 27, 2023, and February 15, 2024 to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of our common stock entitled to vote at the Special Meetings, and as a result, we intend to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time. The Board retains the discretion to determine not to proceed with the Dissolution in its sole discretion and, if it does proceed with the Dissolution, would have discretion as to the timing of the filing of the Certificate of Dissolution. However, if the Board determines that the Dissolution is not in our best interest or in the best interest of our stockholders, the Board may, in its sole discretion, abandon the Dissolution or may amend or modify the Plan of Dissolution to the extent permitted by the Delaware General Corporation Law (the “DGCL”) without the necessity of stockholder approval. After the Certificate of Dissolution has been filed, revocation of the Dissolution would require stockholder approval under the DGCL.
Under Delaware law, utilizing the procedures of Section 281(b) of the DGCL (which is contemplated by the Plan of Dissolution unless otherwise determined by the Board), before a dissolved corporation may make any distribution to its stockholders, it must: (i) pay or make reasonable provision to pay all of its claims and obligations, including all contingent, conditional or unmatured contractual claims known to the corporation, (ii) make such provision as will be reasonably likely to be sufficient to provide compensation for any claim against it which is the subject of a pending action, suit or proceeding to which it is a party, and (iii) make such provision as will be reasonably likely to be sufficient to provide compensation for claims that have not been made known to the corporation or that have not arisen but that, based on facts known to the corporation, are likely to arise or to become known to the corporation within ten years after the date it dissolves. Among other things, our potential liabilities that may require provision could include those relating to indemnification obligations, if any, to third parties or to our current and former officers and directors, and to resolve any stockholder or other litigation that may emerge. It might take significant time to resolve these matters, and as a result we are unable to predict the timing, amount, or number of distributions, if any are made, to our stockholders.
We cannot assure you as to the timing, amount, or number of distributions, if any, to be made to our stockholders.
We cannot predict with certainty the timing, amount, or number of distributions, if any, to our stockholders. Any such amounts may be paid in one or more distributions over a period of several years. Any such distributions will not occur until after the Certificate of Dissolution is filed, and we cannot predict the timing, amount, or number of any such distributions, or whether any such distributions will occur, as uncertainties as to the ultimate amount and scope of our liabilities, the operating costs and amounts to be set aside for claims, obligations, and provisions during the liquidation and winding-up process, and the related timing to complete such transactions, make it impossible to predict with certainty the actual net cash amount, if any, that will ultimately be available for distribution to stockholders or the timing of any such distributions. Examples of uncertainties that could reduce the value of distributions to our stockholders include: the incurrence by the Company of expenses relating to the Dissolution being different than estimated; the receipt of no, or lower than
expected, proceeds in the course of our efforts to monetize our remaining assets and intellectual property; unanticipated costs relating to the defense, satisfaction or settlement of lawsuits or other claims that may be threatened against us or our current or former directors or officers; amounts necessary to resolve claims of any creditors or other third parties; and delays in the Dissolution or other winding-up process.
In addition, as we wind down, we will continue to incur expenses from operations, including directors’ and officers’ insurance, severance payments, payments to service providers and any continuing employees or consultants, taxes, legal, accounting and consulting fees, costs associated with patent prosecution and transitioning this responsibility back to our licensors, expenses related to our filing obligations with the SEC and/or others, and costs associated with continuing to seek approval to dissolve, which will reduce any amounts available for distribution to our stockholders. As a result, we cannot assure you as to any amounts, if any, to be distributed to our stockholders if the Board proceeds with the Dissolution. Because our stockholders did not approve the Dissolution and the Plan of Dissolution, we are not currently able to proceed with the Dissolution and no liquidating distributions will be made in connection therewith, until and unless the Company is able to obtain stockholder or judicial approval to dissolve the Company.
It is the current intent of the Board, assuming approval of the Dissolution, that any cash will first be used to pay our outstanding current liabilities and obligations, and then will be retained to pay ongoing corporate and administrative costs and expenses associated with winding down the Company, liabilities and potential liabilities relating to or arising out of any litigation matters and potential liabilities relating to our indemnification obligations, if any, to our service providers, or to our current and former officers and directors, before such cash, if any remains, will be available for distribution to stockholders.
The Board will determine, in its sole discretion, the timing and number of the distributions of the remaining amounts, if any, to our stockholders in the Dissolution. We can provide no assurance as to if or when any such distributions will be made, and we cannot provide any assurance as to the amount to be paid to stockholders in any such distributions, if any are to be made. Stockholders may receive substantially less than the amount that we currently estimate that they may receive, or they may receive no distribution at all.
The Board may determine not to proceed with the Dissolution.
The Board may determine in its sole discretion not to proceed with the Dissolution, especially if some other alternative emerges, which we do not expect, that would provide greater value to the stockholders than Dissolution and the Plan of Dissolution. If our Board elects to pursue any alternative to the Plan of Dissolution, our stockholders may not receive any of the funds that might otherwise have been available now or in the future for distribution to our stockholders. After the Certificate of Dissolution has been filed, revocation of the Dissolution would require stockholder approval under the DGCL.
Our stockholders may be liable to third parties for part or all of the amount received from us in our liquidating distributions if cash reserves are inadequate.
If the Dissolution becomes effective, we are required to establish a cash reserve designed to satisfy any additional claims and obligations that may arise. Any reserve may not be adequate to cover all of our claims and obligations. Under the DGCL, if we fail to create an adequate reserve for payment of our expenses, claims, and obligations, each stockholder could be held liable for payment to our creditors for claims brought prior to or after the Effective Time (or such longer period as the Delaware Court of Chancery may direct) (the “Survival Period”) (or, if we choose the Safe Harbor Procedures under DGCL Section 280 and 281(a), for claims brought prior to the expiration of the Survival Period), up to the lesser of (i) such stockholder’s pro rata share of amounts owed to creditors in excess of the reserve and (ii) the amounts previously received by such stockholder in the Dissolution from us and from any liquidating trust or trusts. Accordingly, in such event, a stockholder could be required to return part or all of the distributions previously made to such stockholder, and a stockholder could ultimately receive nothing from us under the Plan of Dissolution. Moreover, if a stockholder
has paid taxes on amounts previously received, a repayment of all or a portion of such amount could result in a situation in which a stockholder may incur a net tax cost if the repayment of the amount previously distributed does not cause a commensurate reduction in taxes payable in an amount equal to the amount of the taxes paid on amounts previously distributed.
Our stockholders of record will not be able to buy or sell shares of our common stock after we close our stock transfer books at the Effective Time of the Dissolution.
If the Board determines to proceed with the Dissolution, we intend to close our stock transfer books and discontinue recording transfers of our common stock at the Effective Time of the Dissolution. After we close our stock transfer books, we will not record any further transfers of our common stock on our books except at our sole discretion by will, intestate succession, or operation of law. Therefore, shares of our common stock will not be freely transferable after the Effective Time. As a result of the closing of the stock transfer books, all liquidating distributions in the Dissolution will likely be made to the same stockholders of record as the stockholders of record as of the Effective Time.
We plan to initiate steps to exit from certain reporting requirements under the Exchange Act, which may substantially reduce publicly available information about us. If the exit process is protracted, we will continue to bear the expense of being a public reporting company despite having no source of revenue.
Our common stock is currently registered under the Exchange Act, which requires that we, and our officers and director with respect to Section 16 of the Exchange Act, comply with certain public reporting and proxy statement requirements thereunder. Compliance with these requirements is costly and time-consuming. We plan to initiate steps to exit from such reporting requirements in order to curtail expenses; however, such process may be protracted and we may be required to file Current Reports on Form 8-K or other reports to disclose material events, including those related to the Dissolution. Accordingly, we will continue to incur expenses that will reduce any amount available for distribution, including expenses of complying with public company reporting requirements and paying our service providers, among others. If our reporting obligations cease, publicly available information about us will be substantially reduced.
The loss of key personnel could adversely affect our ability to efficiently dissolve, liquidate, and wind down.
We intend to rely on a few individuals in key management roles and as contractor support to dissolve, liquidate our remaining assets, and wind-down operations, which will continue for at least three years during the Survival Period. Loss of one or more of these key individuals, or inability to contract with essential personnel, could hamper the efficiency or effectiveness of these processes.
We may not be able to find a purchaser for our remaining non-cash assets during the Dissolution.
We own several non-cash assets, including but not limited to preclinical and clinical data packages that we generated in developing FRTX-02 and other product candidates. We may try to find a buyer for these assets but there may be no buyers forthcoming or the offers for the assets may not be adequate. As such there may be no opportunity for any additional distribution to stockholders for these retained assets including during the Survival Period or thereafter.
Stockholders may not be able to recognize a loss for U.S. federal income tax purposes until they receive a final distribution from us.
As a result of the Dissolution, for U.S. federal income tax purposes, a stockholder that is a U.S. person generally will recognize gain or loss on a share-by-share basis equal to the difference between (1) the sum of the amount of cash and the fair market value of property, if any, distributed to the stockholder with respect to each share, less any known liabilities assumed by the stockholder or to which the distributed property (if any) is subject,
and (2) the stockholder’s adjusted tax basis in each share of our common stock. A liquidating distribution pursuant to the Plan of Dissolution may occur at various times and in more than one tax year. Any loss generally will be recognized by a stockholder only in the tax year in which the stockholder receives our final liquidating distribution, and then only if the aggregate value of all liquidating distributions with respect to a share of our common stock is less than the stockholder’s tax basis for that share. Stockholders are urged to consult with their own tax advisors as to the specific tax consequences to them of the Dissolution pursuant to the Plan of Dissolution.
The tax treatment of any liquidating distribution may vary from stockholder to stockholder, and the discussions in this proxy statement regarding tax consequences are general in nature.
We have not requested a ruling from the Internal Revenue Service with respect to the anticipated tax consequences of the Dissolution, and we will not seek an opinion of counsel with respect to the anticipated tax consequences of any liquidating distributions. If any of the anticipated tax consequences described in this Annual Report prove to be incorrect, the result could be increased taxation at the corporate or stockholder level, thus reducing the benefit to our stockholders and/or us from the Dissolution. Tax considerations applicable to particular stockholders may vary with and be contingent on the stockholder’s individual circumstances.
Risks Related to Our Liquidity, Financial Matters, and Our Common Stock
We are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by geopolitical conflict in and around Ukraine, Israel, the broader Middle East, and other areas of the world. Our business, financial condition, and results of operations may be materially adversely affected by the negative impact on the global economy and capital markets resulting from these geopolitical conflicts or other geopolitical tensions.
U.S. and global markets are experiencing volatility and disruption following the escalation of geopolitical tensions and conflict in and around Ukraine, Israel, the broader Middle East, and other areas of the world. Although the length and impact of ongoing military conflicts are highly unpredictable, these conflicts have led to market disruptions, including significant volatility in commodity prices, credit and capital markets, as well as supply chain disruptions. Russian military actions and the resulting sanctions could further adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets.
The extent and duration of the military action, sanctions, and resulting market disruptions are impossible to predict, but could be substantial. Any such disruptions may also magnify the impact of other risks described in this Annual Report.
Our operating results and liquidity needs could be affected negatively by global market fluctuations and economic downturns.
Our operating results and liquidity could be affected negatively by global economic conditions generally, both in the U.S. and elsewhere around the world, including but not limited to that related to geopolitical conflict in and around Ukraine, Israel, the broader Middle East, and other areas of the world, global IT threats, and elevated interest rates. Domestic and international equity and debt markets are experiencing and may in the future experience heightened volatility and turmoil based on domestic and international economic conditions and concerns. In the event these economic conditions and concerns continue or worsen and the markets remain volatile, or an economic recession occurs, our operating results and liquidity could be affected adversely in many ways, making it more difficult for us to operate, and our stock price may decline.
Our stock price and volume of shares traded have been and may continue to be highly volatile, and our common stock may continue to be illiquid.
The market price of our common stock has been subject to significant fluctuations. Market prices for securities of biotechnology and other life sciences companies historically have been particularly volatile and subject to large daily price swings. In addition, there has been limited liquidity in the trading market for our securities, which may adversely affect stockholders. Some of the factors that may cause the market price of our common stock to continue to fluctuate include, but are not limited to:
•the payment of any distribution to stockholders as part of the Dissolution while our outstanding common stock continues to be listed on the OTC Pink market;
•material developments in, or the conclusion of, any litigation to enforce or defend any intellectual property rights or defend against the intellectual property rights of others; and
•the entry into, or termination of, or breach by us or our partners of material agreements, including key commercial partner or licensing agreements.
Moreover, the stock markets in general have experienced substantial volatility in our industry, especially for microcap biotechnology companies, and such volatility has often been unrelated to the operating performance of individual companies or a certain industry segment, such as the ongoing reaction of global markets to geopolitical conflicts and other economic disruptions or concerns, including inflation and interest rate increases. These broad market fluctuations may also adversely affect the trading price of our common stock.
In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our reputation and could expose us to liability or negatively impact our business, financial condition, and operating results.
We are a “smaller reporting company” and the reduced disclosure and governance requirements applicable to smaller reporting companies may make our common stock less attractive to some investors.
We qualify as a “smaller reporting company” under Rule 12b-2 of the Exchange Act. As a smaller reporting company, we are entitled to rely on certain exemptions and reduced disclosure requirements, such as simplified executive compensation disclosures and reduced financial statement disclosure requirements, in our SEC filings. These exemptions and decreased disclosures in our SEC filings due to our status as a smaller reporting company may make it harder for investors to analyze our results of operations and financial prospects. We cannot predict if investors will find our common stock less attractive because we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our common stock price may be more volatile.
We do not anticipate paying any dividends in the foreseeable future.
Our current expectation is that we will retain any future earnings to maximize intended distributions of all remaining cash to stockholders, pending stockholder or judicial approval of the Dissolution and the Plan of Dissolution.
Our ability to use our net operating loss carryforwards and other tax assets to offset future taxable income may be subject to certain limitations.
As of December 31, 2023, we had approximately $432.7 million of federal and $452.3 million of state net operating loss (“NOL”) carryforwards available to offset any future taxable income, of which $217.4 million will carryforward indefinitely and the remainder will expire in varying amounts beginning in 2024 for federal and state purposes if unused. Utilization of these NOLs depends on many factors, including our future income, which cannot be assured. Under the U.S. Tax Cuts and Jobs Acts, U.S. federal NOLs incurred in 2018 and later years may be carried forward indefinitely, but our ability to utilize such U.S. federal NOLs to offset taxable income is limited to 80% of the current-year taxable income. In addition, under Sections 382 and 383 of the Internal Revenue Code of 1986 and corresponding provisions of state law, if a corporation undergoes an “ownership change” (which is generally defined as a greater than 50 percentage points change (by value) in its equity ownership over a rolling three-year period), the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax attributes to offset its post-change income or taxes may be limited. We have not determined whether we have experienced Section 382 ownership changes in the past and if a portion of our NOLs is therefore subject to an annual limitation under Section 382. Therefore, we cannot provide any assurance that a change in ownership within the meaning of the Internal Revenue Code of 1986 and corresponding provisions of state law has not occurred in the past, and there is a risk that changes in ownership could have occurred. We may experience ownership changes as a result of subsequent changes in our stock ownership, which may be outside of our control. In that case, the ability to use NOL carryforwards to offset any future taxable income will be limited following any such ownership change and could be eliminated. If eliminated, the related asset would be removed from the deferred tax asset schedule with a corresponding reduction in the valuation allowance on our financial statements.
Risks Related to Legal, Regulatory, and Compliance Matters
Our business and operations would suffer in the event of system failures, illegal stock trading or manipulation by external parties, cyber-attacks, or a deficiency in or exploitation of our cyber-security.
We rely on cloud-based software to provide the functionality necessary to operate our company, utilizing what is known as “software as a service” (“SaaS”). SaaS allows users like us to connect to and use cloud-based applications over the Internet, such as email, calendaring, and office tools. SaaS provides us with a complete software solution that we purchase on a subscription basis from a cloud service provider. Despite our efforts to protect confidential and sensitive information from unauthorized disclosure across all our platforms, and similar efforts by our cloud service provider(s) and our other third-party contractors, consultants, and vendors, whether information technology (“IT”) providers or otherwise, including but not limited to law firms accountants, and government regulators, this information, and the systems used to store and transmit it, are vulnerable to damage from computer viruses, unauthorized access, computer hacking or breaches, natural disasters, epidemics and pandemics, terrorism, war, labor unrest, and telecommunication and electrical failures. The risk of a security breach or disruption, particularly through cyber-attacks or cyber-intrusion, or other illegal acts, including by computer hackers, foreign governments, and cyber-terrorists, has generally increased as the number, intensity, and sophistication of attempted attacks and intrusions from around the world have increased. Other emerging threats we face include: phishing, account takeover attacks, data breach or theft (no matter where the data are stored), loss of control, especially in SaaS applications, over which users have access to what data and level of access, new malware, zero-day threats, and threats within our own organization. In addition, malicious cyber actors may increase malware and ransom campaigns and phishing emails targeting teleworkers as well as company systems, global conflicts like with Ukraine, Israel, and the broader Middle East, or other world trends and events, which exposes us to additional cybersecurity risks, or may try to illegally obtain material inside information to manipulate our stock price. If such an event were to occur and cause interruptions in our operations, or substantial manipulation of our stock price, it could result in a material disruption of our business operations. In addition, since we have sponsored clinical trials, any breach that compromises patient data and identities, thereby causing a breach of privacy, could generate significant reputational damage and legal
liabilities and costs to recover and repair. For example, the loss or theft of clinical trial data from completed clinical trials could result in stock manipulation and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications or inappropriate disclosure of confidential or proprietary information, we could incur liability or suffer from stock price volatility or decline.
We may face product liability exposure, and if successful claims are brought against us, we may incur substantial liability if our insurance coverage for those claims is inadequate.
We face an inherent risk of product liability or similar causes of action as a result of the clinical testing (and use) of our product candidates or product candidates we have previously sub-licensed, sold, and/or assigned. This risk exists even if a product is manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our products and product candidates, past and present, are designed to affect important bodily functions and processes. Any side effects, manufacturing defects, misuse, or abuse associated with our product candidates could result in actual or perceived injury to a patient that may or may not be reversible or potentially even cause death. We cannot offer any assurance that we will not face product liability or other similar suits in the future or that we will be successful in defending them, nor can we assure that our insurance coverage will be sufficient to cover our liability under any such cases.
In addition, a liability claim may be brought against us even if our product candidates merely appear to have caused an injury. If we cannot successfully defend against product liability or similar claims, we will incur substantial liabilities, reputational harm, and possibly injunctions and punitive actions. In addition, regardless of merit or eventual outcome, product liability claims may result in:
•impairment of our business reputation;
•substantial costs of any related litigation or similar disputes;
•distraction of management’s attention and other resources; or
•substantial monetary awards to patients or other claimants against us that may not be covered by insurance.
Large judgments have been awarded in class action or individual lawsuits based on drugs that had unanticipated side effects. Our insurance coverage may not be sufficient to cover all of our product liability-related expenses or losses and may not cover us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, restrictive, and narrow, and, in the future, we may not be able to maintain adequate insurance coverage at a reasonable cost, or through self-insurance, in sufficient amounts or upon adequate terms to protect us against losses due to product liability or other similar legal actions. A successful product liability claim or series of claims brought against us could, if judgments exceed our insurance coverage, decrease our cash, expose us to liability and harm our business, financial condition, and operating results and lessen the amount, timing, and number of any distributions to stockholder pursuant to the Plan of Dissolution.
We are and may be subject to strict healthcare laws, regulation, and enforcement, and our failure to comply with those laws could expose us to liability or adversely affect our business, financial condition, and operating results.
Certain federal and state healthcare laws and regulations pertaining to patients’ rights and privacy, as well as other rights and obligations, are and may be applicable to our business. We are subject to regulation by the federal government and the states where we or our partners conduct business. The healthcare laws and regulations that may affect our ability to operate include: the Federal Food, Drug and Cosmetic Act, as amended; Title 21 of the Code of Federal Regulations Part 202 (21 CFR Part 202); and civil monetary penalty
laws; federal and state disclosure laws; the Foreign Corrupt Practices Act as it applies to activities both inside and outside of the U.S.; and state law equivalents of many of the above federal laws.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, healthcare reform legislation has strengthened these laws.
Achieving and sustaining compliance with these laws may prove costly. In addition, any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, fluctuation in our stock price, and divert our management’s attention from the operation of our business and result in reputational damage. If our operations are found to be in violation of any of the laws described above or any other governmental laws or regulations that apply to us, we may be subject to penalties, including administrative, civil, and criminal penalties, damages, including punitive damages, fines, disgorgement, individual imprisonment or corporate criminal liability, and injunctions, any of which could expose us to liability and could adversely affect our business, financial condition, and operating results.
Our employees, independent contractors, consultants, vendors, and any partners with which we may collaborate or have collaborated may engage or may have engaged in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, officers, directors, independent contractors, consultants, advisors, vendors, and any partners with which we may collaborate or have collaborated may engage or may have engaged in fraudulent or other illegal or unethical activity. Misconduct by these persons could include intentional, reckless, gross, or negligent misconduct or unauthorized activity that violates: laws or regulations, including those laws requiring the reporting of true, complete, and accurate information to the FDA or foreign regulatory authorities; manufacturing standards; federal, state and foreign laws on data and patient privacy; anticorruption laws, securities laws, and/or laws that require the true, complete and accurate reporting of financial information or data, books, and records. If any such or similar actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal, and administrative and punitive penalties, damages, monetary fines, contractual damages, reputational harm, and injunctions, any of which could expose us to liability and adversely affect our business, financial condition, and operating results and ability to implement the Dissolution and Plan of Dissolution.
We incur costs and demands upon management because of complying with the laws and regulations affecting public companies.
We incur significant legal, accounting, and other expenses and management demands to operate as a public company, including costs associated with public company reporting and other SEC requirements. We also incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act, and rules implemented by the SEC. These rules and regulations have, and are expected to continue to, increase our legal and financial compliance costs and make some activities more time-consuming and costly. These rules and regulations may also make it expensive for us to operate our business and implement the Dissolution and Plan of Dissolution.
Risks Related to Our Intellectual Property
We could be subject to litigation related to our intellectual property and related license agreements for patent infringement under certain circumstances.
Because we rely on certain third-party licensors, licensees, assignees, and partners around the world, if one of our licensors, licensees, assignees, or partners is sued for infringing a third party’s intellectual property rights,
this could expose us to liability, and our business, financial condition, and operating results could suffer in the same manner as if we were sued directly. In addition to facing litigation risks, we have agreed to indemnify certain third-party licensors, licensees, assignees, and partners against claims of infringement caused by our proprietary technologies, and we have entered into cost-sharing agreements with some of our licensors, licensees, assignees, and partners that could require us to pay some of the costs of patent or other intellectual property rights litigation brought against those third parties whether or not the alleged infringement is caused by our proprietary technologies or in-licensed technologies. In certain instances, these cost-sharing agreements could also require us to assume greater responsibility for infringement damages than would be assumed just on the basis of our technology. The occurrence of any of the foregoing could expose us to liability or adversely affect our business, financial condition, and operating results at any time.
General Risk Factors
Provisions of Delaware law and our restated certificate of incorporation and amended and restated bylaws may discourage another company from acquiring us or some or all of our assets and may prevent attempts by our stockholders to replace or remove our current management.
Provisions of Delaware law and our restated certificate of incorporation and amended and restated bylaws may discourage, delay, or prevent a merger, reverse merger, licensing, acquisition, or other strategic transaction that our stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace or remove the sole member of our Board. These provisions include, but are not limited to:
•authorizing the issuance of “blank check” preferred stock without any need for action by stockholders;
•requiring supermajority stockholder voting to effect certain amendments to our current certificate of incorporation and bylaws;
•eliminating the ability of stockholders to call special meetings of stockholders; and
•establishing advance notice requirements for nominations for election to our Board or for proposing matters that can be acted on by stockholders at stockholder meetings.
Although we believe these provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our Board, they would apply even if an offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it difficult for stockholders to replace the sole member of our Board, which is responsible for appointing the members of our management.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
Risk Management and Strategy
We use a “cloud-only” environment for our information systems; there is no central access point that provides access to all software and related data. We use third-party clinical research organizations (“CROs”) to manage all patient-related data and review their cybersecurity procedures as part of vendor evaluation. Our employees do not have access to any patient-identifiable data.
The Company has an IT Security Policy that establishes IT security and rules for the Company. This policy covers both good practice quality guidelines and regulations (“GxP”) and non-GxP IT systems. With the assistance of a senior IT consultant, management has evaluated the potential cyber risk associated with each of our information systems, both internal and external, taken appropriate steps to mitigate risks, and assigned specific tasks to our Cyber Incident Response Team members in case of a cyber incident. Our Cyber Incident Response Team currently consists of all remaining members of management.
Our cybersecurity environment emphasizes the role of each individual user in preventing a cyber incident. We have implemented required monthly video-based cybersecurity training. All users are tested on each training module. This training is supplemented by “test” phishing messages to see if users are alert to cyber risks.
While we have experienced cybersecurity incidents and expect to continue to be subject to such incidents, to date, we have not experienced any cybersecurity incidents that have materially affected our business strategy, results of operations or financial condition. However, we are subject to ongoing risks from cybersecurity threats that could materially affect us, as further described in Part I, Item 1A, “Risk Factors” in this Annual Report. Governance
The Board is responsible for general risk oversight. The Board reviews and evaluates management’s evaluation and mitigation of cyber risks as part of its oversight of the Company’s Risk Management program. Management periodically reviews cyber risks, incidents, and risk mitigation plans and activities with the Board.
In addition to recommendations from our senior IT consultant, we engaged an outside consulting firm to conduct a review of our information systems environment and make recommendations to improve security where appropriate. Management shared the report’s findings with the Board and periodically updates the Board regarding our progress on implementing the report’s recommendations.
ITEM 2. PROPERTIES
We maintain our corporate offices at 2000 Central Avenue, Suite 100, Boulder, CO 80301 under a virtual office lease. We lease our corporate office premises under a monthly membership agreement at a nominal cost. We consider our current office space adequate for our current operations.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our Company, nor is any such litigation threatened as of the date of this filing.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on the over-the-counter market and has been listed on the OTC Pink tier of the OTC Markets under the symbol “FRTX” since December 19, 2023. Quotations of our common stock on the OTC Pink reflect inter-dealer prices, without retail mark-up, mark-down, or commission and may not necessarily represent actual transactions. Our common stock was previously traded on The Nasdaq Capital Market under the symbol “FRTX.”
Holders
As of March 15, 2024, we had 130 registered holders of record of our common stock. A greater number of holders of our common stock are “street name” or beneficial holders, whose shares of record are held by banks, brokers, other financial institutions, and registered clearing agencies.
Stock Repurchases
There were no repurchases made by us or on our behalf, or by any “affiliated purchaser,” of shares of our common stock during the year ended December 31, 2023.
Dividend Policy
We historically have not, and do not anticipate in the future, paying dividends on our common stock. We are not subject to any legal restrictions respecting the payment of dividends, except that we may not pay dividends if the payment would render us insolvent. Subject to these limitations, any future determination as to the payment of cash dividends on our common stock will be at our Board’s discretion and will depend on our financial condition, operating results, capital requirements, and other factors that our Board considers to be relevant.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
On September 19, 2023, we announced the Plan of Dissolution and our intent to discontinue all clinical and preclinical development programs and reduce our workforce. Historically, we were a clinical-stage pharmaceutical company striving to transform patient lives through the development of innovative and differentiated prescription therapeutics. Our pipeline aimed to disrupt existing treatment paradigms and featured several new chemical entities that inhibit novel targets with first-in-class potential for autoimmune, inflammatory, and other debilitating diseases.
Our Board and executive management team conducted a comprehensive process to explore and evaluate strategic alternatives with the goal of maximizing stockholder value. Potential alternatives that were under evaluation included, but were not limited to, a financing, a merger or reverse merger, the sale of all or part of the Company, licensing of assets, a business combination, and/or other strategic transactions or series of related transactions involving the Company.
On September 18, 2023, after conducting an extensive, months-long potential strategic alternatives process, including four unsuccessful attempts to find a merger or reverse merger partner due to the potential acquirer’s inability to secure its own necessary financing and/or inability to offer adequate value to consummate the transaction, and combined with the unsuccessful outreach to approximately 125 other possible counterparties and investors who operate or invest in both life sciences and other industry sectors, our Board unanimously approved the Dissolution and the Plan of Dissolution, subject to the approval of our stockholders. In connection with the Plan of Dissolution, effective October 2, 2023, we discontinued all clinical and preclinical development programs and terminated most of our employees, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company.
We held Special Meetings on November 16, 2023, November 30, 2023, December 15, 2023, December 27, 2023, and February 15, 2024 to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of our common stock entitled to vote at the Special Meetings, and as a result, we intend to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time.
Research and Development Assets
The following image summarizes our current or previous research and development assets, corresponding stage of development, and potential therapeutic areas for each program:
FRTX-02: A Potential First-in-Class Oral DYRK1A Inhibitor for the Treatment of Autoimmune and Inflammatory Diseases
FRTX-02 is a novel, potent, highly selective, and orally bioavailable potential first-in-class, small molecule DYRK1A inhibitor that aims to restore immune balance in patients whose immune systems have become dysregulated. FRTX-02 was our lead development-stage program and has demonstrated promising results in various preclinical and clinical models, including potentially for AD and rheumatoid arthritis.
FRTX-10: A covalent Stimulator of Interferon Genes (STING) inhibitor for the Potential Treatment of Autoimmune, Inflammatory, and Rare Genetic Diseases
FRTX-10 is an early-stage STING inhibitor candidate and a novel, potent, and orally bioavailable covalent STING inhibitor that specifically targets the palmitoylation site of STING. STING is a well-known mediator of innate immune responses. Excessive signaling through STING is linked to numerous high unmet-need diseases,
ranging from autoimmune disorders, such as systemic lupus erythematosus, to interferonopathies, which are a set of rare genetic conditions characterized by interferon overproduction and could have orphan drug potential. Effective March 1, 2024, the license to develop FRTX-10 was terminated by mutual agreement.
Next-Generation Kinase Inhibitors: A Cutting-Edge Platform with the Potential to Produce Treatments for Autoimmune, Inflammatory, and Other Debilitating Diseases
We have exclusive global rights to a cutting-edge platform of next-generation kinase inhibitors. This library of new chemical entities includes next-generation DYRK1 inhibitors, as well as other molecules that specifically inhibit LRRK2, CLK, and TTK, also known as Monopolar spindle 1 (Mps1) kinases. A number of these drug candidates have the potential to penetrate the blood-brain barrier, presenting an opportunity to address neuroinflammatory conditions of high unmet need, such as Down Syndrome, Alzheimer’s Disease, and Parkinson’s Disease, while other peripherally acting novel LRRK2, TTK, and CLK kinase inhibitors could be developed in additional therapeutic areas within autoimmunity, inflammation, and oncology.
Strategic, Licensing, and Other Arrangements
License and Development Agreement with Voronoi
In August 2021, we entered into the Voronoi License Agreement with Voronoi, pursuant to which we acquired exclusive, worldwide rights to research, develop, and commercialize FRTX-02 and other next-generation kinase inhibitors.
With respect to FRTX-02, the Voronoi License Agreement provides that we will make payments to Voronoi of up to $211.0 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. With respect to the compounds arising from the next-generation kinase inhibitor platform, we will make payments to Voronoi of up to $107.5 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. Further, the Voronoi License Agreement provides that we will pay Voronoi tiered royalty payments ranging from low-single digits up to 10% of net sales of products arising from the DYRK1A inhibitor programs and next-generation kinase inhibitor platform. All of the contingent payments and royalties are payable in cash in U.S. Dollars, except for $1.0 million of the development and regulatory milestone payments, which amount is payable in equivalent shares of our common stock. Under the terms of the Voronoi License Agreement, we are responsible for, and bear the future costs of, all development and commercialization activities, including the first right to prosecute and maintain patents, related to all the licensed compounds. As of December 31, 2023 and through the date of this Annual Report, we have not yet made any payments or recorded any liabilities related to the specified development, regulatory, and commercial milestones or royalties on net sales pursuant to the Voronoi License Agreement.
The Voronoi License Agreement also provides that upon termination of the Voronoi License Agreement, Voronoi will be entitled to receive a non-exclusive license to any information and know-how independently developed by the Company for FRTX-02 and other licensed assets in consideration for payment(s) at an arms’-length royalty rate on net sales that must be negotiated in good faith between the parties.
Exclusive License and Development Agreement with Carna
In February 2022, we entered into the Carna License Agreement with Carna, pursuant to which we acquired exclusive, worldwide rights to research, develop, and commercialize Carna’s portfolio of novel STING inhibitors. In accordance with the terms of the Carna License Agreement, in exchange for the licensed rights, we made a one-time cash payment of $2.0 million, which was recorded as research and development expenses in the consolidated statements of operations during the year ended December 31, 2022.
The Carna License Agreement provided that we would make success-based payments to Carna of up to $258.0 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. Further, the Carna License Agreement provided that we would pay Carna tiered royalty payments ranging from mid-single digits up to 10% of net sales. Under the terms of the Carna License Agreement, we were responsible for all development and commercialization activities, including patenting, related to all the licensed compounds. As of December 31, 2023 and through the date of this Annual Report, we have not made any payments or recorded any liabilities related to the specified development, regulatory, and commercial milestones or royalties on net sales pursuant to the Carna License Agreement.
Effective March 1, 2024, the Carna License Agreement was terminated by mutual agreement.
Agreements with Botanix
Asset Purchase Agreement with Botanix
On the Effective Date, we and Brickell Subsidiary entered into the Asset Purchase Agreement with Botanix, pursuant to which Botanix acquired and assumed control of all rights, title, and interests to the Assets. Prior to the sale of the Assets, we had previously entered into the Amended and Restated License Agreement with Bodor, dated December 15, 2012 (last amended in February 2020) that provided us with a worldwide exclusive license to develop, manufacture, market, sell, and sublicense products containing sofpironium bromide through which the Assets were developed. As a result of the Asset Purchase Agreement, Botanix became responsible for all further research, development, and commercialization of sofpironium bromide globally and replaced us as the exclusive licensee under the Amended and Restated License Agreement.
In accordance with the sublicense rights provided to us under the Amended and Restated License Agreement, we also had previously entered into the Kaken Agreement with Kaken, dated as of March 31, 2015 (as amended in May 2018), under which we granted to Kaken an exclusive right to develop, manufacture, and commercialize the sofpironium bromide compound in the Territory. In exchange for the sublicense, we were entitled to receive aggregate payments of up to $10.0 million upon the achievement of specified development milestones, which were earned and received in 2017 and 2018, and up to $19.0 million upon the achievement of sales-based milestones, as well as tiered royalties based on a percentage of net sales of licensed products in the Territory. In September 2020, Kaken received regulatory approval in Japan to manufacture and market ECCLOCK for the treatment of primary axillary hyperhidrosis, and as a result, we began recognizing royalty revenue earned on a percentage of net sales of ECCLOCK in Japan. Pursuant to the Asset Purchase Agreement, the Kaken Agreement was assigned to Botanix, which replaced us as the exclusive sub-licensor to Kaken. During the year ended December 31, 2022, prior to entering into the Asset Purchase Agreement, we recognized royalty revenue under the Kaken Agreement of $0.1 million.
We determined that the development of and ultimate sale and assignment of rights to the Assets is an output of our ordinary activities and Botanix is a customer as it relates to the sale of the Assets and related activities.
On July 21, 2023, we and Brickell Subsidiary entered into the Asset Purchase Agreement Amendment with Botanix. The Asset Purchase Agreement Amendment provided that, in lieu of any remaining Post-Closing Payment Obligations, Botanix would pay $6.6 million to us and $1.7 million on behalf of us to Bodor. The payments from Botanix to the Company and Bodor were made on July 26, 2023. The Asset Purchase Agreement Amendment also provided that upon payment of the amounts by Botanix thereunder, all Post-Closing Payment Obligations under the Asset Purchase Agreement were terminated and of no further force or effect.
In accordance with the terms of the Asset Purchase Agreement, in exchange for the Assets, we (i) received an upfront payment at closing in the amount of $3.0 million, (ii) were reimbursed for certain recent development expenditures in advancement of the Assets, (iii) received a milestone payment of $2.0 million upon the
acceptance by the FDA in December 2022 of the filing of an NDA for sofpironium bromide gel, 15%, and (iv) would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, a contingent milestone payment of $4.0 million if marketing approval in the U.S. for sofpironium bromide gel, 15%, had been received on or before September 30, 2023, or $2.5 million if such marketing approval had been received after September 30, 2023 but on or before February 17, 2024. Botanix submitted an NDA for sofpironium bromide gel, 15%, to the FDA in September 2022, which was accepted for filing by the FDA in December 2022. Under the Asset Purchase Agreement, we also would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, additional success-based regulatory and sales milestone payments of up to $168.0 million. Further, we would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, tiered Earnout Payments.
The Asset Purchase Agreement also provided that Botanix would pay us Sublicense Income. Sublicense Income represented our estimate of payments that would be earned by us in the applicable period from sales-based milestone payments and royalties Botanix would receive from Kaken to the extent it was probable that a significant reversal in the amount of cumulative revenue recognized would not occur. Royalties vary based on net sales that are impacted by a wide variety of market and other factors. We recorded a contract asset equal to the amount of revenue recognized related to the Sublicense Income, less the amount of payments received from or due by Botanix in relation to the Sublicense Income. As a result of entering into the Asset Purchase Agreement Amendment, the contract asset was fully settled.
All other consideration due under the Asset Purchase Agreement was contingent upon certain regulatory approvals and future sales subsequent to such regulatory approvals, or was based upon future sales that we determined were not yet probable due to such revenues being highly susceptible to factors outside of our influence and uncertainty about the amount of such consideration that would not be resolved for an extended period of time. Therefore, we determined that such variable consideration amounts were fully constrained up through the date of the Asset Purchase Agreement Amendment, and, as such, did not recognize such amounts as contract revenue.
Transition Services Agreement with Botanix
In connection with the sale of the Assets, on the Effective Date, we and Botanix entered into the TSA whereby we provide consulting services as an independent contractor to Botanix in support of and through filing and potential approval of the U.S. NDA for sofpironium bromide gel, 15%. In accordance with the terms of the TSA, in exchange for providing these services (i) prior to the acceptance of the filing by the FDA of such NDA in December 2022, we received from Botanix a fixed monthly amount of $71 thousand, and (ii) after the acceptance of the filing in December 2022, we receive from Botanix a variable amount based upon actual hours worked, in each case plus related fees and expenses of our advisors (plus a 5% administrative fee) and our out-of-pocket expenses. As of the date of this Annual Report, we do not expect to provide any further services or receive any additional fees related to the TSA.
Contract Revenue under the Botanix Agreements
The Company recognized the following as contract revenue (in thousands):
| | | | | | | | | | | | | | | |
| | | Year Ended
December 31, |
| | | | | 2023 | | 2022 |
| Buyout of Post-Closing Payment Obligations | | | | | $ | 7,944 | | | $ | — | |
| Sublicense Income | | | | | 53 | | | 433 | |
| Consulting services provided under the TSA | | | | | 9 | | | 794 | |
| Upfront consideration | | | | | — | | | 3,000 | |
| Milestone payment received upon acceptance by FDA of NDA filing | | | | | — | | | 2,000 | |
| Reimbursed development expenditures under the Asset Purchase Agreement | | | | | — | | | 624 | |
| Total contract revenue | | | | | $ | 8,006 | | | $ | 6,851 | |
Agreements with Bodor
In connection with the sale of the Assets, on the Effective Date, we, Brickell Subsidiary, and Bodor entered into the Rights Agreement to clarify that we and Brickell Subsidiary have the power and authority under the Amended and Restated License Agreement to enter into the Asset Purchase Agreement and the TSA, and that Botanix would assume the Amended and Restated License Agreement pursuant to the Asset Purchase Agreement. The Rights Agreement included a general release of claims and no admission of liability between the parties. Pursuant to such Rights Agreement, as subsequently amended on November 10, 2022, we agreed to pay Bodor (i) 20% of the amount of each payment due to us from Botanix for upfront and milestone payments, subject to deductions, credits, or offsets applied under the Asset Purchase Agreement, as well as (ii) certain tiered payments, set as a percentage ranging from mid-single digits to mid-teen digits, of the amount of each of the applicable Earnout Payments due to us from Botanix after deductions, credits, or offsets applied under the Asset Purchase Agreement.
Pursuant to the terms of the Asset Purchase Agreement, we retained our obligation under the Amended and Restated License Agreement to issue $1.0 million in shares of our common stock to Bodor upon the FDA’s acceptance of an NDA filing for sofpironium bromide gel, 15%. On November 10, 2022, we entered into the Acknowledgment with Brickell Subsidiary, Botanix, and Bodor. Pursuant to the Acknowledgment, we paid $1.0 million in cash to Bodor in full satisfaction of our obligation to issue shares upon the FDA’s acceptance of the NDA. We determined to prepay this obligation in cash in order to avoid the substantial dilution to our stockholders that would have resulted if we had issued the shares of our common stock originally provided for in the Amended and Restated License Agreement.
In connection with the Asset Purchase Agreement Amendment, on July 21, 2023, we, Brickell Subsidiary, and Bodor entered into the RA Amendment. The RA Amendment provides that in exchange for the one-time payment of $1.7 million by Botanix on behalf of us to Bodor, we shall have no further payment obligations to Bodor under or in connection with the Rights Agreement or the Amended and Restated License Agreement. Except as explicitly amended by the RA Amendment, the Rights Agreement remains in full force and effect.
During the year ended December 31, 2023, we incurred $1.7 million of general and administrative expenses associated with payments due to Bodor. During the year ended December 31, 2022, $1.9 million of general and administrative expenses were associated with achieved milestones or payments due to Bodor related to sofpironium bromide gel, 15%. Prior to the execution of the Rights Agreement, we paid Bodor immaterial amounts with respect to the royalties we received from Kaken for sales of ECCLOCK in Japan during those periods.
Common Stock Listing
Our common stock was previously traded on The Nasdaq Capital Market under the symbol “FRTX” until it was delisted from Nasdaq on December 18, 2023. Beginning December 19, 2023, our common stock began trading on the OTC Pink tier of the OTC Markets under the symbol “FRTX.”
Significant Financing Arrangements
This section sets forth our recent and ongoing financing arrangements, all of which involve our common stock.
Public Offerings of Common Stock and Warrants
In October 2020, we completed the sale of 422,300 shares of our common stock, and, to certain investors, pre-funded warrants to purchase 40,663 shares of our common stock and accompanying common stock warrants to purchase up to an aggregate of 462,979 shares of our common stock (the “October 2020 Offering”). The October 2020 Offering resulted in net proceeds of approximately $13.7 million to us after deducting underwriting commissions and discounts and other offering expenses payable by us of $1.3 million and excluding the proceeds from the exercise of the warrants. The common warrants provide the warrant holder with the right to participate in distributions on a 1-for-1 basis with common shareholders. No warrants associated with the October 2020 Offering were exercised during the years ended December 31, 2023 or 2022.
In June 2020, we completed the sale of 328,669 shares of our common stock, and, to certain investors, pre-funded warrants to purchase 60,220 shares of our common stock and accompanying common stock warrants to purchase up to an aggregate of 388,920 shares of our common stock (the “June 2020 Offering”). The June 2020 Offering resulted in approximately $18.7 million of net proceeds after deducting underwriting commissions and discounts and other offering expenses payable by us of $1.4 million and excluding the proceeds from the exercise of the warrants. The common warrants provide the warrant holder with the right to participate in distributions on a 1-for-1 basis with common shareholders. No warrants associated with the June 2020 Offering were exercised during the years ended December 31, 2023 or 2022.
For additional information regarding the offerings described above, see Note 6. “Capital Stock” of the notes to our consolidated financial statements included in this Annual Report.
At Market Issuance Sales Agreements
In March 2021, we entered into an At Market Issuance Sales Agreement (the “2021 ATM Agreement”) with Oppenheimer & Co. Inc. (“Oppenheimer”) and William Blair & Company, L.L.C. as our sales agents (the “Agents”). The 2021 ATM Agreement was subsequently terminated effective December 22, 2023. Pursuant to the terms of the 2021 ATM Agreement, we could sell from time to time through the Agents shares of our common stock having an aggregate offering price of up to $50.0 million. Sales of shares were made by means of ordinary brokers’ transactions on The Nasdaq Capital Market at market prices or as otherwise agreed by us and the Agents. Under the terms of the 2021 ATM Agreement, we could also sell the shares from time to time to an Agent as principal for its own account at a price to be agreed upon at the time of sale. Any sale of the shares to an Agent as principal would have been sold pursuant to the terms of a separate placement notice between us and such Agent. During the year ended December 31, 2023, we sold 2,887,535 shares of common stock under the 2021 ATM Agreement at a weighted-average price of $2.34 per share, for aggregate net proceeds of $6.6 million, after giving effect to a 3% commission to the Agents. During the year ended December 31, 2022, we sold 354,381 shares of common stock under the 2021 ATM Agreement at a weighted-average price of $3.70 per share, for aggregate net proceeds of $1.3 million, after giving effect to a 3% commission to the Agents. As of the date the 2021 ATM Agreement was terminated, approximately $38.0 million of shares of common stock were remaining, but had not yet been sold under the 2021 ATM Agreement.
In April 2020, we entered into an At Market Issuance Sales Agreement (the “2020 ATM Agreement”) with Oppenheimer as our sales agent. The 2020 ATM Agreement was subsequently terminated effective December 22, 2023. Pursuant to the terms of the 2020 ATM Agreement, we could sell from time to time through Oppenheimer shares of our common stock having an aggregate offering price of up to $8.0 million. During the years ended December 31, 2023 and 2022, no sales of common stock under the 2020 ATM Agreement occurred. As of the date the 2020 ATM Agreement was terminated, approximately $2.6 million of shares of common stock were remaining, but had not yet been sold by the Company under the 2020 ATM Agreement.
Private Placement Offerings
In February 2020, we and Lincoln Park Capital Fund, LLC (“Lincoln Park”) entered into (i) a securities purchase agreement (the “Securities Purchase Agreement”); (ii) a purchase agreement (the “Purchase Agreement”); and (iii) a registration rights agreement. Pursuant to the Securities Purchase Agreement, Lincoln Park purchased, and we sold, (i) an aggregate of 21,111 shares of common stock (the “Common Shares”); (ii) a warrant to initially purchase an aggregate of up to 13,476 shares of common stock at an exercise price of $0.45 per share (the “Series A Warrant”); and (iii) a warrant to initially purchase an aggregate of up to 34,588 shares of common stock at an exercise price of $52.20 per share (the “Series B Warrant” and, together with the Series A Warrant, the “Warrants”). The aggregate gross purchase price for the Common Shares and the Warrants was $2.0 million. The Warrants provide the warrant holder with the right to participate in distributions on a 1-for-1 basis with common shareholders. No Warrants associated with the Securities Purchase Agreement were exercised during the years ended December 31, 2023 or 2022.
Under the terms and subject to the conditions of the Purchase Agreement, we had the right, but not the obligation, to sell to Lincoln Park, and Lincoln Park was obligated to purchase, up to $28.0 million in the aggregate of shares of our common stock. On September 1, 2023, the Purchase Agreement expired according to its terms. During the years ended December 31, 2023 and 2022, no sales of common stock under the Purchase Agreement occurred.
Financial Overview
Our operations to date have been limited to business planning, raising capital, developing and entering into strategic partnerships for our pipeline assets, identifying, in-licensing and out-licensing and/or selling product candidates, conducting clinical trials, and other research and development activities.
To date, we have financed operations primarily through funds received from the sale of common stock and warrants, convertible preferred stock, debt and convertible notes, and payments received under license, collaboration, and other agreements. Other than through arrangements as they relate to sales of ECCLOCK in Japan, none of our product candidates has been approved for sale and we have not generated any product sales. Since inception, we have incurred operating losses with the exception of the three months ended September 30, 2023. We recorded a net loss of $5.7 million and $21.1 million for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $172.2 million. We expect to continue to incur additional expenses and operating losses for the foreseeable future as we implement the Plan of Dissolution.
On September 18, 2023, after conducting an extensive, months-long potential strategic alternatives process, including four unsuccessful attempts to find a merger or reverse merger partner due to the potential acquirer’s inability to secure its own necessary financing and/or inability to offer adequate value to consummate the transaction, and combined with the unsuccessful outreach to approximately 125 other possible counterparties and investors who operate or invest in both life sciences and other industry sectors, our Board unanimously approved the Dissolution and the Plan of Dissolution, subject to the approval of the Company’s stockholders. We held Special Meetings on November 16, 2023, November 30, 2023, December 15, 2023, December 27,
2023, and February 15, 2024 to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of our common stock entitled to vote at the Special Meetings, and as a result, we intend to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time. Additionally, in order to reduce costs, we have discontinued all clinical and preclinical development programs and reduced our workforce, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company.
Key Components of Operations
Revenue
Revenue generally consists of revenue recognized under our strategic agreements for the development and commercialization of our product candidates. Our strategic agreements generally outline overall development plans and include payments we receive at signing, payments for the achievement of certain milestones, sublicense income, earnout payments on net product sales, and royalties on net product sales. For these activities and payments, we utilize judgment to assess the nature of the performance obligations to determine whether the performance obligations are satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. Prior to entering into the Asset Purchase Agreement, we recognized royalty revenue earned on a percentage of net sales of ECCLOCK in Japan. Beginning in the second quarter of 2022, we began recognizing contract revenue pursuant to the terms of the Asset Purchase Agreement. After entering into the Asset Purchase Agreement Amendment in July 2023 and recognizing $7.9 million in revenue from Botanix on July 27, 2023, we do not expect to generate any further significant revenue from any product candidates that we developed under the Asset Purchase Agreement or Asset Purchase Agreement Amendment.
Research and Development Expenses
Research and development expenses principally consist of payments to CROs and upfront in-licensing fees for development-stage assets. CROs help plan, organize, and conduct clinical and nonclinical studies under our direction. Personnel costs, including wages, benefits, and share-based compensation, related to our research and development staff in support of product development activities are also included, as well as costs incurred for supplies, clinical and nonclinical studies, consultants, and facility and related overhead costs.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel costs, including wages, benefits, share-based compensation, and severance, related to our executive, sales, marketing, finance, and human resources personnel, as well as professional fees, including legal, accounting, and licensing fees.
Total Other Income, Net
Other income, net consists primarily of interest income, interest expense, and various income or expense items of a non-recurring nature. We have earned interest income from money market funds and interest-bearing accounts. Our interest income varies each reporting period depending on our average cash balances during the period and market interest rates. We expect interest income to fluctuate in the future with changes in average cash balances and market interest rates.
Critical Accounting Estimates
We have prepared the consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of these consolidated financial
statements requires us to make estimates, assumptions, and judgments that affect the reported amounts of assets, liabilities, and related disclosures at the date of the consolidated financial statements, and the reported amounts of revenue and expenses during the reporting period. On an ongoing basis, management evaluates its critical estimates, including those related to revenue recognition and accrued research and development expenses. We base our estimates on our historical experience and on assumptions that we believe are reasonable; however, actual results may differ materially from these estimates under different assumptions or conditions.
For information on our significant accounting policies, please refer to Note 2 of the notes to our consolidated financial statements included elsewhere in this Annual Report.
Contract Revenue Recognition
Pursuant to the Asset Purchase Agreement, and prior to the Asset Purchase Agreement Amendment in July 2023, we had rights to receive from Botanix future milestone payments, sales-based payments, and sublicense income related to sales-based milestone payments and royalties earned by Botanix from Kaken under the Kaken Agreement (all of such payments, “Botanix Payments”). The payments under the Asset Purchase Agreement varied based on net sales and/or were contingent upon certain regulatory approvals. Therefore, we were required to estimate the Botanix Payments, which represented variable consideration, to be achieved and recognized revenue to the extent it was probable that a significant reversal in the amount of cumulative revenue recognized would not occur. We used either the most likely amount or the expected value method in making such estimates based on the nature of the payments to be received and whether there was a wide range of outcomes or only two possible outcomes. For any milestone payments, we utilized the most likely amount method, which represented our best estimate of the single most likely outcome to be achieved. For any sales-based payments or other consideration where there were more than two possible outcomes, we utilized the expected value method, which represented the sum of probability-weighted amounts in a range of possible consideration amounts.
We based our estimates of variable consideration to be recognized as revenue using the applicable method described above on factors such as, but not limited to, required regulatory approvals, historical sales levels, market events and projections, and others as necessary. We updated our estimates at each reporting period based on actual results and future expectations as necessary. Our estimates were subject to changes in net sales of sofpironium bromide and the occurrence of contingent events, such as regulatory approvals. Changes in net sales could occur due to various risks such as competitors entering the market, technology changes as to how hyperhidrosis was treated, and foreign exchange risk. After entering into the Asset Purchase Agreement Amendment in July 2023 and recognizing $7.9 million in revenue from Botanix, we were not eligible to receive any further Botanix Payments.
Research and Development
Research and development costs are charged to expense when incurred and consist of costs incurred for independent and collaboration research and development activities. The major components of research and development costs include formulation development, nonclinical studies, clinical studies, clinical manufacturing costs, in-licensing fees for development-stage assets, salaries and employee benefits, and allocations of various overhead and occupancy costs. Research costs typically consist of applied research, preclinical, and toxicology work. Pharmaceutical manufacturing development costs consist of product formulation, chemical analysis, and the transfer and scale-up of manufacturing at contract manufacturers. Assets acquired (or in-licensed) that are utilized in research and development that have no alternative future use are expensed as incurred. Milestone payments related to our acquired (or in-licensed) assets are recorded as research and development expenses when probable and reasonably estimable.
Costs for certain research and development activities, such as clinical trial expenses, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, and information provided to us by our vendor on their actual costs incurred or level of effort
expended. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected on the consolidated balance sheets as prepaid expenses and other current assets or accrued expenses. As of December 31, 2023, we had no balances reported in the consolidated balance sheet for accrued expenses or prepaid expenses associated with research and development activities.
We have entered into licensing or subscription arrangements to access and utilize certain technology. In each case, we evaluate if the license agreement results in the acquisition of an asset or a business. To date, none of our license agreements have been considered an acquisition of a business. For asset acquisitions, the upfront payments to acquire such licenses, as well as any future milestone payments made before product approval that do not meet the definition of a derivative, are immediately recognized as research and development expenses when they are paid or become payable, provided there is no alternative future use of the rights in other research and development projects.
Recent Accounting Pronouncements
We believe that the impact of recently issued guidance, whether adopted or to be adopted in the future, is not expected to have a material impact on our consolidated financial statements upon adoption.
Results of Operations
As described above, in July 2023, we recognized $7.9 million in revenue pursuant to the Asset Purchase Agreement Amendment with Botanix, and we incurred $1.7 million of general and administrative expenses associated with payments due to Bodor. Further, in connection with the Plan of Dissolution, effective October 2, 2023, we discontinued all clinical and preclinical development programs and terminated most of our employees, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company. As a result, we do not expect the discussions of our historical results of operations below to be indicative of our results of operations in the current or future periods.
Comparison of the Years Ended December 31, 2023 and 2022
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended
December 31, | | |
| | | | | 2023 | | 2022 | | Change |
| | | | | (in thousands) | | |
| Revenue | | | | | $ | 8,006 | | | $ | 6,943 | | | $ | 1,063 | |
| | | | | | | | | |
| | | | | | | | | |
| Research and development expenses | | | | | (3,182) | | | (14,043) | | | 10,861 | |
| General and administrative expenses | | | | | (11,184) | | | (14,434) | | | 3,250 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Other income, net | | | | | 666 | | | 432 | | | 234 | |
Net loss | | | | | $ | (5,694) | | | $ | (21,102) | | | $ | 15,408 | |
Revenue
Revenue increased by approximately $1.1 million for the year ended December 31, 2023 compared to the year ended December 31, 2022. Revenue for the year ended December 31, 2023 primarily consisted of contract revenue of $7.9 million pursuant to the Asset Purchase Agreement Amendment with Botanix, in which we agreed to terminate and relinquish any remaining amounts potentially payable by Botanix to us in the future, and $0.1 million pursuant to the Asset Purchase Agreement and TSA with Botanix. Revenue for the year ended December 31, 2022 primarily consisted of contract revenue recognized under the Asset Purchase Agreement and TSA with Botanix of $6.9 million and royalty revenue of $0.1 million. Contract revenue recorded during the year ended December 31, 2022 consisted of the following: an upfront payment from Botanix of
$3.0 million; a milestone payment of $2.0 million upon the FDA’s acceptance of Botanix’s NDA submission of sofpironium bromide gel, 15%; fees for consulting services we provided under the TSA with Botanix of $0.8 million; reimbursed development expenditures from Botanix under the Asset Purchase Agreement of $0.6 million; and Sublicense Income under the Asset Purchase Agreement of $0.4 million.
Research and Development Expenses
Research and development expenses decreased by $10.9 million for the year ended December 31, 2023 compared to the year ended December 31, 2022, driven primarily by decreased costs of $4.8 million related to the completion in December 2022 of our Phase 1 clinical trial of FRTX-02, $2.1 million related to our Phase 3 pivotal clinical program of sofpironium bromide in 2022 that did not recur, $2.1 million related to acquisition and license costs for our portfolio of STING inhibitor program in 2022 that did not recur, and $1.9 million in lower personnel and other unallocated expenses related to reduced research and development activities in 2023 as well as the reduction in research and development personnel in 2023. Below is a summary of our research and development expenses by period by program:
| | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended
December 31, | | | |
| | | | | 2023 | | 2022 | | | Change |
| | | | | | | | | | |
| | | | | (in thousands) | | | |
| | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| Direct program expenses related to | | | | | | | | | | |
| Sofpironium bromide | | | | | $ | — | | | $ | 2,090 | | | | $ | (2,090) | |
| DYRK1A inhibitor program (FRTX-02) | | | | | 1,261 | | | 6,046 | | | | (4,785) | |
| STING inhibitor program (FRTX-10) | | | | | 111 | | | 2,162 | | | | (2,051) | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| Personnel and other unallocated expenses | | | | | 1,810 | | | 3,745 | | | | (1,935) | |
| Total research and development expenses | | | | | $ | 3,182 | | | $ | 14,043 | | | | $ | (10,861) | |
General and Administrative Expenses
General and administrative expenses decreased by $3.3 million for the year ended December 31, 2023 compared to the year ended December 31, 2022. The decrease of $3.3 million was primarily related to $2.4 million in lower legal and compliance fees, $1.1 million in lower compensation-related expense, $0.7 million in lower insurance and other fees, and $0.3 million in lower licensing fees to Bodor, partially offset by an increase of $1.4 million in severance expense.
Total Other Income, Net
Total other income, net increased by $0.2 million for the year ended December 31, 2023 compared to the year ended December 31, 2022. Total other income, net in the 2023 period primarily consisted of interest income on our investment balances and in the 2022 period primarily consisted of a gain from the extinguishment of liabilities assumed by Botanix related to development costs during the year ended December 31, 2022 prior to the Effective Date of the Asset Purchase Agreement.
Liquidity and Capital Resources
We have incurred significant operating losses and have an accumulated deficit as a result of in-licensing and development of our product candidates, including conducting preclinical and clinical trials and providing general and administrative support for these operations. For the years ended December 31, 2023 and 2022, we had a net loss of $5.7 million and $21.1 million, respectively. As of December 31, 2023, we had an accumulated deficit of $172.2 million. As of December 31, 2023, we had cash and cash equivalents of $10.9 million. Since
inception, we have financed our operations primarily through funds received from the sale of common stock and warrants, convertible preferred stock, debt and convertible notes, and payments received under license and other strategic agreements. We expect to continue to incur additional losses for the foreseeable future as we implement the Plan of Dissolution.
Our Board and executive management team conducted a comprehensive process to explore and evaluate strategic alternatives with the goal of maximizing stockholder value. Potential alternatives that were under evaluation included, but were not limited to, a financing, a merger or reverse merger, the sale of all or part of the Company, licensing of assets, a business combination, and/or other strategic transactions or series of related transactions involving the Company.
On September 18, 2023, after conducting an extensive, months-long potential strategic alternatives process, including four unsuccessful attempts to find a merger or reverse merger partner due to the potential acquirer’s inability to secure its own necessary financing and/or inability to offer adequate value to consummate the transaction, and combined with the unsuccessful outreach to approximately 125 other possible counterparties and investors who operate or invest in both life sciences and other industry sectors, our Board unanimously approved the Dissolution and the Plan of Dissolution, subject to the approval of the Company’s stockholders.
We held Special Meetings on November 16, 2023, November 30, 2023, December 15, 2023, December 27, 2023, and February 15, 2024 to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of our common stock entitled to vote at the Special Meetings, and as a result, we intend to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time. Additionally, in order to reduce costs, we have discontinued all clinical and preclinical development programs and reduced our workforce, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company.
As a result of the Plan of Dissolution, our management has concluded that substantial doubt exists about our ability to continue as a going concern for a period of twelve months from the date of issuance of our accompanying consolidated financial statements, which do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty. Accordingly, the consolidated financial statements have been prepared on a basis that assumes that we will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
Cash Flows
Since inception, we have primarily used our available cash to fund expenditures related to efforts to in-license and develop our product candidates, including conducting preclinical and clinical trials and providing general
and administrative support for these operations. The following table sets forth a summary of cash flows for the periods presented:
| | | | | | | | | | | |
| | | |
| Year Ended
December 31, |
| 2023 | | 2022 |
| | | |
| | | |
| (in thousands) |
| Net cash provided by (used in): | | | |
| Operating activities | $ | (4,343) | | | $ | (19,335) | |
| Investing activities | — | | | (47) | |
| Financing activities | 6,531 | | | 1,178 | |
| Total | $ | 2,188 | | | $ | (18,204) | |
Operating Activities
Net cash used in operating activities of $4.3 million during the year ended December 31, 2023 decreased compared to $19.3 million during the year ended December 31, 2022, primarily as a result of a decrease in cash used to support our operating activities, including but not limited to our clinical trials, research and development activities, and general working capital requirements. The $15.0 million decrease was impacted by the net effect of a decrease in net loss, adjusted for non-cash items of $15.2 million and the net effect of changes in working capital of $0.2 million.
Financing Activities
Net cash provided by financing activities during the year ended December 31, 2023 increased by $5.4 million compared to the year ended December 31, 2022, primarily resulting from increased net proceeds received during the year ended December 31, 2023 of $5.4 million from sales of our common stock under the 2021 ATM Agreement.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information under this item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Fresh Tracks Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Fresh Tracks Therapeutics, Inc. (the Company) as of December 31, 2023 and 2022, the related consolidated statements of operations, redeemable preferred stock and stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
The Company's Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company is seeking approval to dissolve and distribute all remaining cash to stockholders and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management's evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the Board of Directors and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. We determined that there are no critical audit matters.
| | | | | | | | | | | | | | |
/s/ Ernst & Young LLP |
|
| We have served as the Company’s auditor since 2017. |
|
| Denver, Colorado |
|
| March 15, 2024 |
FRESH TRACKS THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| | | |
| | | |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 10,868 | | | $ | 8,680 | |
| | | |
| Prepaid expenses and other current assets | 684 | | | 1,403 | |
| Total current assets | 11,552 | | | 10,083 | |
| Property and equipment, net | 34 | | | 75 | |
| Contract asset, net of current portion | — | | | 64 | |
| Operating lease right-of-use asset | — | | | 49 | |
| | | |
| Total assets | $ | 11,586 | | | $ | 10,271 | |
Liabilities and stockholders’ equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 406 | | | $ | 571 | |
| Accrued liabilities | 1,250 | | | 2,457 | |
| Lease liability | — | | | 49 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Total current liabilities | 1,656 | | | 3,077 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Commitments and contingencies (Note 5) | | | |
| | | |
| | | |
| Stockholders’ equity: | | | |
Common stock, $0.01 par value, 300,000,000 shares authorized as of December 31, 2023 and 2022; 5,973,306 and 3,018,940 shares issued and outstanding as of December 31, 2023 and 2022, respectively | 60 | | | 30 | |
| Additional paid-in capital | 182,033 | | | 173,633 | |
| | | |
| Accumulated deficit | (172,163) | | | (166,469) | |
| Total stockholders’ equity | 9,930 | | | 7,194 | |
| Total liabilities and stockholders’ equity | $ | 11,586 | | | $ | 10,271 | |
| | | |
See accompanying notes to these consolidated financial statements.
FRESH TRACKS THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | |
| | | Year Ended
December 31, |
| | | | | 2023 | | 2022 |
| Revenue | | | | | | | |
| Contract revenue | | | | | $ | 8,006 | | | $ | 6,851 | |
| Royalty revenue | | | | | — | | | 92 | |
| Total revenue | | | | | 8,006 | | | 6,943 | |
| | | | | | | |
| Operating expenses: | | | | | | | |
| Research and development | | | | | 3,182 | | | 14,043 | |
| General and administrative | | | | | 11,184 | | | 14,434 | |
| Total operating expenses | | | | | 14,366 | | | 28,477 | |
Loss from operations | | | | | (6,360) | | | (21,534) | |
| Other income | | | | | 671 | | | 441 | |
| | | | | | | |
| Interest expense | | | | | (5) | | | (9) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Net loss attributable to common stockholders | | | | | $ | (5,694) | | | $ | (21,102) | |
| | | | | | | |
Net loss per common share attributable to common stockholders, basic and diluted | | | | | $ | (1.06) | | | $ | (7.51) | |
| | | | | | | |
Weighted-average shares used to compute net loss per common share attributable to common stockholders, basic and diluted | | | | | 5,394,551 | | | 2,808,075 | |
| | | | | | | |
See accompanying notes to these consolidated financial statements.
FRESH TRACKS THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF REDEEMABLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY
(in thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Series A Redeemable Preferred Stock | | Common Stock | | Additional
Paid-In-Capital | | | | Accumulated
Deficit | | Total
Stockholders’
Equity |
| Shares | | Par Value | | Shares | | Par Value | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | — | | | $ | — | | | 2,652,828 | | | $ | 27 | | | $ | 170,247 | | | | | $ | (145,367) | | | $ | 24,907 | |
| Issuance of preferred stock | 1 | | | — | | | — | | | — | | | — | | | | | — | | | — | |
| Redemption of preferred stock | (1) | | | — | | | — | | | — | | | — | | | | | — | | | — | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
Issuance of common stock pursuant to ATM agreement, net of issuance costs of $117 | — | | | — | | | 354,381 | | | 3 | | | 1,193 | | | | | — | | | 1,196 | |
| | | | | | | | | | | | | | | |
| Issuance of common stock for cash under employee stock purchase plan | — | | | — | | | 11,731 | | | — | | | 37 | | | | | — | | | 37 | |
| Stock-based compensation | — | | | — | | | — | | | — | | | 2,156 | | | | | — | | | 2,156 | |
| | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | — | | | — | | | | | (21,102) | | | (21,102) | |
| Balance, December 31, 2022 | — | | | — | | | 3,018,940 | | | 30 | | | 173,633 | | | | | (166,469) | | | 7,194 | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
Common stock issued pursuant to ATM agreement, net of issuance costs of $202 | — | | | — | | | 2,887,535 | | | 29 | | | 6,540 | | | | | — | | | 6,569 | |
| Issuance of common stock upon restricted stock unit settlement, net of shares withheld for taxes | — | | | — | | | 66,831 | | | 1 | | | (39) | | | | | — | | | (38) | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Repurchase of restricted stock units | — | | | — | | | — | | | — | | | (20) | | | | | — | | | (20) | |
| Stock-based compensation | — | | | — | | | — | | | — | | | 1,919 | | | | | — | | | 1,919 | |
| | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | — | | | — | | | | | (5,694) | | | (5,694) | |
| Balance, December 31, 2023 | — | | | $ | — | | | 5,973,306 | | | $ | 60 | | | $ | 182,033 | | | | | $ | (172,163) | | | $ | 9,930 | |
See accompanying notes to these consolidated financial statements.
FRESH TRACKS THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | |
| Year Ended
December 31, |
| 2023 | | 2022 |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | |
| Net loss | $ | (5,694) | | | $ | (21,102) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Stock-based compensation | 1,919 | | | 2,156 | |
| Non-cash operating lease expense | 60 | | | 59 | |
| Depreciation | 41 | | | 30 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Changes in operating assets and liabilities: | | | |
| Prepaid expenses and other current assets, including noncurrent portion of contract asset | 783 | | | 1,249 | |
| Accounts payable | (165) | | | (1,034) | |
| Accrued liabilities | (1,227) | | | (624) | |
| Operating lease liability | (60) | | | (69) | |
| | | |
| | | |
| | | |
| | | |
| Net cash used in operating activities | (4,343) | | | (19,335) | |
| | | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | |
| Capital expenditures | — | | | (47) | |
| | | |
| | | |
| Net cash used in investing activities | — | | | (47) | |
| | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | |
| | | |
| | | |
| Proceeds from the issuance of common stock pursuant to ATM agreement, net of issuance costs | 6,569 | | | 1,196 | |
| Payments of taxes related to net share settlement of equity awards | (38) | | | (55) | |
| Proceeds from the issuance of common stock under employee stock purchase plan | — | | | 37 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Net cash provided by financing activities | 6,531 | | | 1,178 | |
| | | |
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 2,188 | | | (18,204) | |
| CASH AND CASH EQUIVALENTS—BEGINNING | 8,680 | | | 26,884 | |
| CASH AND CASH EQUIVALENTS—ENDING | $ | 10,868 | | | $ | 8,680 | |
| | | |
| | | |
| | | |
| Supplemental Disclosure of Non-Cash Investing and Financing Activities: | | | |
| Acquisition of right-of-use asset through lease liability | $ | 11 | | | $ | 49 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
See accompanying notes to these consolidated financial statements.
FRESH TRACKS THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. ORGANIZATION AND NATURE OF OPERATIONS
On September 19, 2023, Fresh Tracks Therapeutics, Inc. (the “Company” or “Fresh Tracks”) announced a proposed plan of liquidation and dissolution (the “Plan of Dissolution”) and its intent to discontinue all clinical and preclinical development programs and reduce its workforce. Historically, the Company was a clinical-stage pharmaceutical company striving to transform patient lives through the development of innovative and differentiated prescription therapeutics. The Company’s pipeline aimed to disrupt existing treatment paradigms and featured several new chemical entities that inhibit novel targets with first-in-class potential for autoimmune, inflammatory, and other debilitating diseases. In connection with the Plan of Dissolution, effective October 2, 2023, the Company discontinued all clinical and preclinical development programs and terminated most of its employees, except for certain employees, consultants, and advisors who will supervise and facilitate the dissolution and wind down of the Company.
Liquidity and Capital Resources
The Company has incurred significant operating losses and has an accumulated deficit as a result of in-licensing and development of product candidates, including conducting preclinical and clinical trials and providing general and administrative support for these operations. For the year ended December 31, 2023, the Company had a net loss of $5.7 million and net cash used in operating activities of $4.3 million. As of December 31, 2023, the Company had cash and cash equivalents of $10.9 million and an accumulated deficit of $172.2 million. The Company expects to continue to incur additional losses for the foreseeable future as it implements the Plan of Dissolution.
During the year ended December 31, 2023, the Company’s board of directors (“Board”) and executive management team conducted a comprehensive process to explore and evaluate strategic alternatives with the goal of maximizing stockholder value. Potential alternatives that were under evaluation included, but were not limited to, a financing, a merger or reverse merger, the sale of all or part of the Company, licensing of assets, a business combination, and/or other strategic transactions or series of related transactions involving the Company.
On September 18, 2023, the Board unanimously approved the liquidation and dissolution of the Company (the “Dissolution”) and the Plan of Dissolution, subject to the approval of the Company’s stockholders. The Company held special meetings of stockholders on November 16, 2023, November 30, 2023, December 15, 2023, December 27, 2023, and February 15, 2024 (the “Special Meetings”) to seek stockholder approval of the Dissolution and the Plan of Dissolution. However, the Dissolution and Plan of Dissolution did not receive the affirmative vote of a majority of the outstanding shares of the Company’s common stock entitled to vote at the Special Meetings, and as a result, the Company intends to continue to seek approval to dissolve and distribute all remaining cash to stockholders over time.
As a result of the Plan of Dissolution, management has concluded that substantial doubt exists about the Company’s ability to continue as a going concern for a period of twelve months from the date of issuance of the consolidated financial statements, which do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty about the ability to continue as a going concern. Accordingly, the consolidated financial statements have been prepared on a basis that assumes the Company will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business. If the Company obtains approval to dissolve and the likelihood is remote that the Company
would return from liquidation, the Company would consider liquidation to be imminent and apply liquidation basis of accounting.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Brickell Subsidiary, Inc. (“Brickell Subsidiary”), and are presented in United States (“U.S.”) dollars and prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which include all adjustments necessary for the fair presentation of the Company’s financial position, results of operations, and cash flows for the periods presented. All significant intercompany balances have been eliminated in consolidation. The Company operates in one operating segment and, accordingly, no segment disclosures have been presented herein.
Use of Estimates
The Company’s consolidated financial statements are prepared in accordance with U.S. GAAP, which requires it to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Although these estimates are based on the Company’s knowledge of current events and actions it may take in the future, actual results may ultimately differ from these estimates and assumptions.
Risks and Uncertainties
The Company’s business is subject to significant risks, including, but not limited to, uncertainty of plans and expectations for the Dissolution and the Plan of Dissolution and the scope, timing, rate of progress, and expense of the Company’s ongoing and future activities; the ongoing liquidity of the Company’s outstanding common stock; the Company’s ability, if there is interest from potential purchasers, to sell any of its assets as part of the Plan of Dissolution, including but not limited to independently developed data packages, technology, and other intellectual property; compliance with regulatory and other legal requirements; and ability to manage business partners and other alliances, including potential return of product licenses and termination of these and other existing contractual relationships with the Company.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less from date of purchase to be cash equivalents. Cash equivalents consist primarily of amounts held in short-term money market accounts with highly rated financial institutions.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and accounts receivable. The Company maintains cash and cash equivalents balances in several accounts with one financial institution which, from time to time, are in excess of federally insured limits.
One third party individually accounted for all of the Company’s revenue for the years ended December 31, 2023 and 2022, as well as associated accounts receivable and contract asset balances as of December 31, 2022. Refer to Note 3. “Strategic Agreements” for a detailed discussion of agreements with Botanix SB Inc. and Botanix
Pharmaceuticals Limited (“Botanix”) and the termination of the Company’s future payment rights in exchange for a single up-front payment in the year ended December 31, 2023.
Property and Equipment
Property and equipment is stated at cost, less accumulated depreciation. Expenditures for major betterments and additions are charged to the asset accounts, while replacements, maintenance, and repairs, which do not improve or extend the lives of the respective assets, are charged to expense as incurred. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally between three and five years. Depreciation expense amounted to $41 thousand and $30 thousand for the years ended December 31, 2023 and 2022, respectively.
Fair Value Measurements
Fair value is the price that the Company would receive to sell an asset or pay to transfer a liability in a timely transaction with an independent counterparty in the principal market, or in the absence of a principal market, the most advantageous market for the asset or liability. A three-tier hierarchy distinguishes between (1) inputs that reflect the assumptions market participants would use in pricing an asset or liability developed based on market data obtained from sources independent of the reporting entity (observable inputs) and (2) inputs that reflect the reporting entity’s own assumptions about the assumptions market participants would use in pricing an asset or liability developed based on the best information available in the circumstances (unobservable inputs). The hierarchy is summarized in the three broad levels listed below:
Level 1—quoted prices in active markets for identical assets and liabilities
Level 2—other significant observable inputs (including quoted prices for similar assets and liabilities, interest rates, credit risk, etc.)
Level 3—significant unobservable inputs (including the Company’s own assumptions in determining the fair value of assets and liabilities)
The following table sets forth the fair value of the Company’s financial assets measured at fair value on a recurring basis based on the three-tier fair value hierarchy (in thousands):
| | | | | | | | | | | | | | | |
| Level 1 | | | | |
| December 31, | | | | |
| 2023 | | 2022 | | | | |
| | | | |
| | | | | | | |
| Money market funds | $ | 10,617 | | | $ | 7,680 | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Fair Value of Financial Instruments
The following methods and assumptions were used by the Company in estimating the fair values of each class of financial instrument disclosed herein:
Money Market Funds—The carrying amounts reported as cash equivalents in the consolidated balance sheets approximate their fair values due to their short-term nature and market rates of interest.
The carrying values of cash equivalents, accounts receivable, accounts payable, and accrued liabilities approximate fair value due to the short-term maturity of those items.
Revenue Recognition
The Company has historically recognized revenue primarily from upfront fees, research and development milestones, research reimbursements, and consulting services fees related to the development of previously owned or sublicensed assets associated with the proprietary compound sofpironium bromide, as well as sublicense income and royalty fees on sales of sofpironium bromide gel, 5% (ECCLOCK®) in Japan.
The Company recognizes revenue upon the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. In determining the appropriate amount of revenue to be recognized, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price, including the constraint on variable consideration; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the Company satisfies the performance obligations. At contract inception, the Company assesses the goods or services promised within each contract and assesses whether each promised good or service is distinct and determines those that are performance obligations. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
The Company utilizes judgment to assess the nature of the performance obligation to determine whether the performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Contract Revenue
The Company evaluates its contracts, including asset sale arrangements that involve the Company’s rights to intellectual property, to determine whether they are outputs of the Company’s ordinary activities and whether the counterparty meets the definition of a customer. If the arrangement is determined to be a contract with a customer and the goods or services sold are determined to be distinct from other performance obligations identified in the arrangement, the Company recognizes revenue primarily from non-refundable upfront fees, milestone payments, sales-based payments, and fees for consulting services allocated to the goods or services when (or as) control is transferred to the customer, and the customer can use and benefit from the goods or services.
Licenses of Intellectual Property
If a license for the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue when the functional license is transferred to the customer, and the customer can use and benefit from the license.
Milestones
At the inception of each arrangement that includes milestone payments (variable consideration), excluding sales-based milestone payments discussed below, the Company evaluates whether the milestones are considered probable of being reached and estimates the amount to be included in the transaction price using the most likely amount method. The most likely amount method is generally utilized when there are only two possible outcomes and represents the Company’s best estimate of the single most likely outcome to be achieved. If it is probable that a significant revenue reversal would not occur, the variable consideration for the associated milestone is included in the transaction price. Milestone payments contingent on regulatory approvals that are not within the Company or the Company’s collaboration partner’s control, as applicable, are generally not considered probable of being achieved until those approvals are received. At the end of each subsequent
reporting period, the Company re-evaluates the probability of achievement of milestones and any related constraint, and if necessary, adjusts the Company’s estimate of the variable consideration. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue in the period of adjustment.
Sales-Based Payments
For license arrangements that include sales-based payments such as royalties or milestone payments based on the level of sales, and for which the license is deemed to be the predominant item to which the sales-based payments relate, the Company recognizes revenue at the later of (i) when the related sales occur or (ii) when the performance obligation to which some or all of the sales-based payment has been allocated has been satisfied (or partially satisfied). Sales-based payments received under license arrangements are recorded as royalty revenue in the Company’s consolidated statements of operations.
For non-license arrangements that include sales-based payments, including earnout payments and milestone payments based on the level of sales, the Company estimates the sales-based payments (variable consideration) to be achieved and recognizes revenue to the extent it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. The Company may use either the most likely amount, as described above, or the expected value method, in making such estimates based on the nature of the payment to be received and whether there is a wide range of outcomes or only two possible outcomes. The expected value method represents the sum of probability-weighted amounts in a range of possible consideration amounts. The Company bases its estimates using the applicable method described above on factors such as, but not limited to, required regulatory approvals, historical sales levels, market events and projections, and other factors as appropriate. The Company updates its estimates at each reporting period based on actual results and future expectations as necessary.
Contract Asset
For non-license arrangements involving the sale and transfer of the Company’s intellectual property rights, the Company recognizes estimated variable consideration as revenue as discussed above before the customer pays consideration or before payment is due. The estimated revenue recognized is presented as a contract asset on the Company’s consolidated balance sheets. The current portion of the contract asset is presented in prepaid expenses and other current assets on the Company’s consolidated balance sheets. Actual amounts paid or due by the customer are recorded as a reduction to the contract asset. Any revisions to the Company’s estimated revenue based on actual results and future expectations are recognized as an adjustment to the contract asset.
Research and Development
Research and development costs are charged to expense when incurred and consist of costs incurred for independent and collaboration research and development activities. The major components of research and development costs include formulation development, nonclinical studies, clinical studies, clinical manufacturing costs, in-licensing fees for development-stage assets, salaries and employee benefits, and allocations of various overhead and occupancy costs. Research costs typically consist of applied research, preclinical, and toxicology work. Pharmaceutical manufacturing development costs consist of product formulation, chemical analysis, and the transfer and scale-up of manufacturing at contract manufacturers. Assets acquired (or in-licensed) that are utilized in research and development that have no alternative future use are expensed as incurred. Milestone payments related to the Company’s acquired (or in-licensed) assets are recorded as research and development expenses when probable and reasonably estimable.
Costs for certain research and development activities, such as clinical trial expenses, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, and information provided to the Company by its vendor on their actual costs incurred or level of
effort expended. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected on the consolidated balance sheets as prepaid expenses and other current assets or accrued expenses.
When the Company enters into licensing or subscription arrangements to access and utilize certain technology, the Company evaluates if the license agreement results in the acquisition of an asset or a business. To date, none of the Company’s license agreements have been considered an acquisition of a business. For asset acquisitions, the upfront payments to acquire such licenses, as well as any future milestone payments made before product approval that do not meet the definition of a derivative, are immediately recognized as research and development expenses when they are paid or become payable, provided there is no alternative future use of the rights in other research and development projects.
Net Loss per Share
Basic and diluted net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding. When the effects are not anti-dilutive, diluted earnings per share is computed by dividing the Company’s net income by the weighted-average number of common shares outstanding and the impact of all potentially dilutive common shares. Diluted net loss per share is the same as basic net loss per share, as the effects of potentially dilutive securities are anti-dilutive for all periods presented.
The following table sets forth the potential common shares excluded from the calculation of diluted net loss per share because their inclusion would be anti-dilutive:
| | | | | | | | | | | | | | | |
| | | Year Ended
December 31, |
| | | | | 2023 | | 2022 |
| Outstanding common stock warrants | | | | | 621,063 | | | 621,063 | |
| Outstanding options | | | | | — | | | 215,316 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Total | | | | | 621,063 | | | 836,379 | |
Leases
The Company determines if an arrangement is a lease at inception. Operating leases with a term greater than one year are recognized on the consolidated balance sheets as right-of-use assets and lease liabilities. The Company does not hold any finance leases. The Company has elected the practical expedient not to recognize on the consolidated balance sheets leases with terms of one year or less and not to separate lease components and non-lease components for real estate leases. Lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company estimates the incremental borrowing rate in determining the present value of lease payments. Lease expense is recognized on a straight-line basis over the lease term.
Redeemable Preferred Stock
The Company issued one share of redeemable preferred stock in May 2022. The redeemable preferred stock contained provisions that required redemption under circumstances that were outside of the Company’s control and was classified as a mezzanine instrument outside of the Company’s capital accounts. The share of redeemable preferred stock was sold to one investor for $10 and was subsequently redeemed in July 2022, as described further in Note 6. “Capital Stock.”
Income Taxes
The Company accounts for income taxes by using an asset and liability method of accounting for deferred income taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. A valuation allowance is recorded to the extent it is more likely than not that a deferred tax asset will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date.
The Company’s significant deferred tax assets are for net operating loss (“NOL”) carryforwards, tax credits, fixed assets, and intangible assets. The Company has provided a valuation allowance for its entire net deferred tax assets since inception because, due to its history of operating losses, the Company has concluded that it is more likely than not that its deferred tax assets will not be realized.
The Company recognizes interest and penalties arising from the underpayment of income taxes in the consolidated statements of operations as a component of income tax expense. The Company had no accrual for interest or penalties on its consolidated balance sheets as of December 31, 2023 and 2022, and has not recognized interest or penalties in its consolidated statements of operations for the years ended December 31, 2023 and 2022.
New Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standard setting bodies that the Company adopts as of the specified effective date. The Company does not believe that the adoption of recently issued standards has had or will have a material impact on the Company’s consolidated financial statements or disclosures.
NOTE 3. STRATEGIC AGREEMENTS
License and Development Agreement with Voronoi
On August 27, 2021, the Company entered into a License and Development Agreement (the “Voronoi License Agreement”) with Voronoi Inc. (“Voronoi”), pursuant to which the Company acquired exclusive, worldwide rights to research, develop, and commercialize FRTX-02 and other next-generation kinase inhibitors.
With respect to FRTX-02, the Voronoi License Agreement provides that the Company will make payments to Voronoi of up to $211.0 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. With respect to the compounds arising from the next-generation kinase inhibitor platform, the Company will make payments to Voronoi of up to $107.5 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. Further, the Voronoi License Agreement provides that the Company will pay Voronoi tiered royalty payments ranging from low-single digits up to 10% of net sales of products arising from the DYRK1A inhibitor programs and next-generation kinase inhibitor platform. All of the contingent payments and royalties are payable in cash in U.S. Dollars, except for $1.0 million of the development and regulatory milestone payments, which amount is payable in equivalent shares of the Company’s common stock. Under the terms of the Voronoi License Agreement, the Company is responsible for, and bears the future costs of, all development and commercialization activities, including the first right to prosecute and maintain patents, related to all the licensed compounds. As of December 31, 2023 and through the date of this Annual Report, the Company has not yet made any payments or recorded any liabilities related to the specified development, regulatory, and commercial milestones or royalties on net sales pursuant to the Voronoi License Agreement.
The Voronoi License Agreement also provides that upon termination of the Voronoi License Agreement, Voronoi will be entitled to receive a non-exclusive license to any information and know-how independently developed by the Company for FRTX-02 and other licensed assets in consideration for payment(s) at an arms’-length royalty rate on net sales that must be negotiated in good faith between the parties.
Exclusive License and Development Agreement with Carna
On February 2, 2022, the Company entered into an Exclusive License Agreement (the “Carna License Agreement”) with Carna Biosciences, Inc. (“Carna”), pursuant to which the Company acquired exclusive, worldwide rights to research, develop, and commercialize Carna’s portfolio of novel STING inhibitors. In accordance with the terms of the Carna License Agreement, in exchange for the licensed rights, the Company made a one-time cash payment of $2.0 million, which was recorded as research and development expenses in the consolidated statements of operations during the year ended December 31, 2022.
The Carna License Agreement provided that the Company would make success-based payments to Carna of up to $258.0 million in the aggregate contingent upon achievement of specified development, regulatory, and commercial milestones. Further, the Carna License Agreement provided that the Company would pay Carna tiered royalty payments ranging from mid-single digits up to 10% of net sales. Under the terms of the Carna License Agreement, the Company was responsible for all development and commercialization activities, including patenting, related to all the licensed compounds. As of December 31, 2023 and through the date of this Annual Report, the Company has not made any payments or recorded any liabilities related to the specified development, regulatory, and commercial milestones or royalties on net sales pursuant to the Carna License Agreement.
Effective March 1, 2024, the Carna License Agreement was terminated by mutual agreement.
Agreements with Botanix
Asset Purchase Agreement with Botanix
On May 3, 2022 (the “Effective Date”), the Company and Brickell Subsidiary entered into an asset purchase agreement with Botanix (the “Asset Purchase Agreement”), pursuant to which Botanix acquired and assumed control of all rights, title, and interests to assets primarily related to the proprietary compound sofpironium bromide that were owned and/or licensed by the Company or Brickell Subsidiary (the “Assets”). Prior to the sale of the Assets, the Company had previously entered into a License Agreement with Bodor Laboratories, Inc. (“Bodor”), dated December 15, 2012 (last amended in February 2020) that provided the Company with a worldwide exclusive license to develop, manufacture, market, sell, and sublicense products containing sofpironium bromide through which the Assets were developed (the “Amended and Restated License Agreement”). As a result of the Asset Purchase Agreement, Botanix became responsible for all further research, development, and commercialization of sofpironium bromide globally and replaced the Company as the exclusive licensee under the Amended and Restated License Agreement.
In accordance with the sublicense rights provided to the Company under the Amended and Restated License Agreement, the Company also had previously entered into a License, Development, and Commercialization Agreement with Kaken Pharmaceutical Co., Ltd. (“Kaken”), dated as of March 31, 2015 (as amended in May 2018, the “Kaken Agreement”), under which the Company granted to Kaken an exclusive right to develop, manufacture, and commercialize the sofpironium bromide compound in Japan and certain other Asian countries (the “Territory”). In exchange for the sublicense, the Company was entitled to receive aggregate payments of up to $10.0 million upon the achievement of specified development milestones, which were earned and received in 2017 and 2018, and up to $19.0 million upon the achievement of sales-based milestones, as well as tiered royalties based on a percentage of net sales of licensed products in the Territory. In September 2020, Kaken received regulatory approval in Japan to manufacture and market ECCLOCK for the treatment of primary
axillary hyperhidrosis, and as a result, the Company began recognizing royalty revenue earned on a percentage of net sales of ECCLOCK in Japan. Pursuant to the Asset Purchase Agreement, the Kaken Agreement was assigned to Botanix, which replaced the Company as the exclusive sub-licensor to Kaken. During the year ended December 31, 2022, prior to entering into the Asset Purchase Agreement, the Company recognized royalty revenue under the Kaken Agreement of $0.1 million.
The Company determined that the development of and ultimate sale and assignment of rights to the Assets is an output of the Company’s ordinary activities and Botanix is a customer as it relates to the sale of the Assets and related activities.
On July 21, 2023, the Company and Brickell Subsidiary entered into Amendment No. 1 to the Asset Purchase Agreement (the “Asset Purchase Agreement Amendment”) with Botanix. The Asset Purchase Agreement Amendment provided that, in lieu of any remaining amounts potentially payable by Botanix to the Company pursuant to the Asset Purchase Agreement (collectively, the “Post-Closing Payment Obligations”), Botanix would pay $6.6 million to the Company and $1.7 million on behalf of the Company to Bodor. The payments from Botanix to the Company and Bodor were made on July 26, 2023. The Asset Purchase Agreement Amendment also provided that upon payment of the amounts by Botanix thereunder, all Post-Closing Payment Obligations under the Asset Purchase Agreement were terminated and of no further force or effect.
In accordance with the terms of the Asset Purchase Agreement, in exchange for the Assets, the Company (i) received an upfront payment at closing in the amount of $3.0 million, (ii) was reimbursed for certain recent development expenditures in advancement of the Assets, (iii) received a milestone payment of $2.0 million upon the acceptance by the U.S. Food and Drug Administration (“FDA”) in December 2022 of the filing of a new drug application (“NDA”) for sofpironium bromide gel, 15%, and (iv) would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, a contingent milestone payment of $4.0 million if marketing approval in the U.S. for sofpironium bromide gel, 15%, had been received on or before September 30, 2023, or $2.5 million if such marketing approval had been received after September 30, 2023 but on or before February 17, 2024. Botanix submitted an NDA for sofpironium bromide gel, 15%, to the FDA in September 2022, which was accepted for filing by the FDA in December 2022. Under the Asset Purchase Agreement, the Company also would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, additional success-based regulatory and sales milestone payments of up to $168.0 million. Further, the Company would have been eligible to receive, prior to the Asset Purchase Agreement Amendment, tiered earnout payments ranging from high-single digits to mid-teen digits on net sales of sofpironium bromide gel (the “Earnout Payments”).
The Asset Purchase Agreement also provided that Botanix would pay the Company a portion of the sales-based milestone payments and royalties that Botanix received from Kaken under the assigned Kaken Agreement (together, the “Sublicense Income”). Sublicense Income represented the Company’s estimate of payments that would be earned by the Company in the applicable period from sales-based milestone payments and royalties Botanix would receive from Kaken to the extent it was probable that a significant reversal in the amount of cumulative revenue recognized would not occur. Royalties vary based on net sales that are impacted by a wide variety of market and other factors and, as such, the Company utilized the expected value approach, which the Company believed would best predict the amount of consideration to which it would be entitled. In relation to the sales-based milestone payments that Botanix could receive from Kaken in the future, the Company utilized the most likely amount method and determined it was not yet probable that the Company would receive any payments from Botanix in relation to such milestone payments. Therefore, the Company determined that such milestone payments were fully constrained up through the date of the Asset Purchase Agreement Amendment, and, as such, did not recognize such amounts as contract revenue. With respect to the recognition of contract revenue for the Sublicense Income based on future royalties that would be due to Botanix from Kaken, certain amounts were not yet due from Botanix. Therefore, the Company recorded a contract asset equal to the amount of revenue recognized related to the Sublicense Income, less the amount of payments received from or due by
Botanix in relation to the Sublicense Income. As a result of entering into the Asset Purchase Agreement Amendment, the contract asset was fully settled.
All other consideration due under the Asset Purchase Agreement was contingent upon certain regulatory approvals and future sales subsequent to such regulatory approvals, or was based upon future sales that the Company determined were not yet probable due to such revenues being highly susceptible to factors outside of the Company’s influence and uncertainty about the amount of such consideration that would not be resolved for an extended period of time. Therefore, the Company determined that such variable consideration amounts were fully constrained up through the date of the Asset Purchase Agreement Amendment, and as such, did not recognize such amounts as contract revenue.
Transition Services Agreement with Botanix
In connection with the sale of the Assets, on the Effective Date, the Company and Botanix entered into a transition services agreement (the “TSA”) whereby the Company provides consulting services as an independent contractor to Botanix in support of and through filing and potential approval of the U.S. NDA for sofpironium bromide gel, 15%. In accordance with the terms of the TSA, in exchange for providing these services (i) prior to the acceptance of the filing by the FDA of such NDA in December 2022, the Company received from Botanix a fixed monthly amount of $71 thousand, and (ii) after the acceptance of the filing in December 2022, the Company receives from Botanix a variable amount based upon actual hours worked, in each case plus related fees and expenses of the Company’s advisors (plus a 5% administrative fee) and the Company’s out-of-pocket expenses.
Contract Revenue and Contract Asset under the Botanix Agreements
The Company recognized the following as contract revenue (in thousands):
| | | | | | | | | | | | | | | |
| | | Year Ended
December 31, |
| | | | | 2023 | | 2022 |
| Buyout of Post-Closing Payment Obligations | | | | | $ | 7,944 | | | $ | — | |
| Sublicense Income | | | | | 53 | | | 433 | |
| Consulting services provided under the TSA | | | | | 9 | | | 794 | |
| Upfront consideration | | | | | — | | | 3,000 | |
| Milestone payment received upon acceptance by FDA of NDA filing | | | | | — | | | 2,000 | |
| Reimbursed development expenditures under the Asset Purchase Agreement | | | | | — | | | 624 | |
| Total contract revenue | | | | | $ | 8,006 | | | $ | 6,851 | |
The following table presents changes in the value of the Company’s contract asset related to Sublicense Income for the following periods (in thousands):
| | | | | |
| Contract asset as of January 1, 2023 | $ | 318 | |
| |
| Amounts received | (318) | |
| Contract asset as of December 31, 2023 | $ | — | |
| |
| |
| | | | | |
| Contract asset as of January 1, 2022 | $ | — | |
| Sublicense Income recognized | 433 | |
| Amounts received or receivable | (115) | |
Contract asset as of December 31, 2022 | $ | 318 | |
Contract asset, included in prepaid expenses and other current assets | $ | 254 | |
Contract asset, net of current portion | $ | 64 | |
Agreements with Bodor
In connection with the sale of the Assets, on the Effective Date, the Company, Brickell Subsidiary, and Bodor entered into an agreement (the “Rights Agreement”) to clarify that the Company and Brickell Subsidiary have the power and authority under the Amended and Restated License Agreement to enter into the Asset Purchase Agreement and the TSA, and that Botanix would assume the Amended and Restated License Agreement pursuant to the Asset Purchase Agreement. The Rights Agreement included a general release of claims and no admission of liability between the parties. Pursuant to such Rights Agreement, as subsequently amended on November 10, 2022, the Company agreed to pay Bodor (i) 20% of the amount of each payment due to the Company from Botanix for upfront and milestone payments, subject to deductions, credits, or offsets applied under the Asset Purchase Agreement, as well as (ii) certain tiered payments, set as a percentage ranging from mid-single digits to mid-teen digits, of the amount of each of the applicable Earnout Payments due to the Company from Botanix after deductions, credits or offsets applied under the Asset Purchase Agreement.
Pursuant to the terms of the Asset Purchase Agreement, the Company retained its obligation under the Amended and Restated License Agreement to issue $1.0 million in shares of its common stock to Bodor upon the FDA’s acceptance of an NDA filing for sofpironium bromide gel, 15%. On November 10, 2022, the Company entered into an Acknowledgment and Agreement Related to Asset Purchase Agreement and Amended and Restated License Agreement (the “Acknowledgment”) with Brickell Subsidiary, Botanix, and Bodor. Pursuant to the Acknowledgment, the Company paid $1.0 million in cash to Bodor in full satisfaction of the Company’s obligation to issue shares upon the FDA’s acceptance of the NDA.
In connection with the Asset Purchase Agreement Amendment, on July 21, 2023, the Company, Brickell Subsidiary, and Bodor entered into a Second Amendment to Rights Agreement (the “RA Amendment”). The RA Amendment provides that in exchange for the one-time payment of $1.7 million by Botanix on behalf of the Company to Bodor, the Company shall have no further payment obligations to Bodor under or in connection with the Rights Agreement or the Amended and Restated License Agreement.
During the year ended December 31, 2023, the Company incurred $1.7 million of general and administrative expenses associated with payments due to Bodor. During the year ended December 31, 2022, $1.9 million of general and administrative expenses were associated with achieved milestones or payments due to Bodor related to sofpironium bromide gel, 15%. Prior to the execution of the Rights Agreement, the Company paid Bodor immaterial amounts with respect to the royalties the Company received from Kaken for sales of ECCLOCK in Japan during those periods.
NOTE 4. DETAILED ACCOUNT BALANCES
Prepaid expenses and other current assets consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| | | |
| Prepaid insurance | $ | 545 | | | $ | 521 | |
| Accounts receivable | 45 | | | 250 | |
| Contract asset | — | | | 254 | |
| | | |
| Prepaid research and development expenses | — | | | 254 | |
| | | |
| Other | 94 | | | 124 | |
| Total | $ | 684 | | | $ | 1,403 | |
Accrued liabilities consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| | | |
| Accrued severance | $ | 1,018 | | | $ | — | |
| Accrued compensation | 232 | | | 1,320 | |
| Accrued professional fees | — | | | 705 | |
| Accrued research and development expenses | — | | | 432 | |
| | | |
| | | |
| | | |
| | | |
| Total | $ | 1,250 | | | $ | 2,457 | |
NOTE 5. COMMITMENTS AND CONTINGENCIES
Operating Lease
In August 2016, the Company entered into a multi-year, noncancelable lease for its Colorado-based office space, which was amended on December 29, 2022 to, among other things, extend the lease term to December 31, 2025, eliminate options previously available to the Company to extend the lease, and provide that the Company may terminate the lease effective June 30, 2023 if notice is provided by April 30, 2023 (as amended, the “Boulder Lease”). Minimum base lease payments under the Boulder Lease were recognized on a straight-line basis over the term of the lease. In addition to base rental payments included in the contractual obligations table below, the Company was responsible for its pro rata share of the operating expenses for the building, which included common area maintenance, utilities, property taxes, and insurance.
Upon modification of the Boulder Lease in December 2022, the Company reassessed classification of the lease and determined that the lease still met the criteria to be classified as an operating lease. Furthermore, the Company remeasured the lease liability as of the effective date by calculating the present value of the new lease payments, discounted at the Company’s incremental borrowing rate, over the lease term of six months. The lease term included periods covered by an option to terminate the lease that the Company was reasonably certain to exercise. The operating expenses were variable and were not included in the present value determination of the lease liability.
On May 4, 2023, the Company entered into an amendment to the Boulder Lease, which terminated the Boulder Lease, effective August 31, 2023, and provided for the payment of a termination fee by the Company of approximately $5 thousand in May 2023. Upon modification of the Boulder Lease, the Company reassessed classification of the lease and determined that the lease still met the criteria to be classified as an operating lease. Furthermore, the Company remeasured the lease liability as of the effective date by calculating the
present value of the new lease payments, discounted at the Company’s incremental borrowing rate, over the remaining lease term of four months. The operating expenses were variable and were not included in the present value determination of the lease liability.
As of December 31, 2023, the Company had no contractual obligations related to operating lease commitments.
The following table presents information pertaining to the Company’s former operating leases (in thousands):
| | | | | | | | | | | | | | | |
| | | Year Ended
December 31, |
| | | | | 2023 | | 2022 |
| Operating lease cost | | | | | $ | 67 | | | $ | 62 | |
| Variable lease cost | | | | | $ | 20 | | | $ | 39 | |
| | | | | | | |
| Cash outflows from operating leases | | | | | $ | 81 | | | $ | 111 | |
| | | | | | | |
| Weighted-average remaining lease term | | | | | — years | | 0.5 years |
| Weighted-average discount rate | | | | | 11.0 | % | | 11.0 | % |
Licensing and Other Agreements
Refer to Note 3. “Strategic Agreements” for more information about the Company’s obligations under its licensing and other agreements.
NOTE 6. CAPITAL STOCK
Common Stock
Under the Company’s Restated Certificate of Incorporation, the Company’s Board has the authority to issue up to 300,000,000 shares of common stock with a par value of $0.01 per share. Each share of the Company’s common stock is entitled to one vote, and the holders of the Company’s common stock are entitled to receive dividends when and as declared or paid by its Board.
The Company had reserved authorized shares of common stock for future issuance as of December 31, 2023 as follows:
| | | | | |
| December 31,
2023 |
| Common stock warrants | 621,063 | |
| |
| |
| |
| |
| |
Public Offerings of Common Stock and Warrants
In October 2020, the Company completed a sale of 422,300 shares of its common stock, and, to certain investors, pre-funded warrants to purchase 40,663 shares of its common stock and accompanying common stock warrants to purchase up to an aggregate of 462,979 shares of its common stock (the “October 2020 Offering”). Each share of common stock and pre-funded warrant to purchase one share of the Company’s common stock was sold together with a common warrant to purchase one share of the Company’s common stock. The shares of common stock and pre-funded warrants, and the accompanying common warrants, were issued separately and were immediately separable upon issuance. The common warrants are exercisable at a price of $32.40 per share of the Company’s common stock and will expire five years from the date of issuance. The common warrants provide the warrant holder with the right to participate in distributions on a 1-for-1 basis with common shareholders. The pre-funded warrants were exercised in October 2020. No warrants associated with the October 2020 Offering were exercised during the years ended December 31, 2023 or 2022.
In June 2020, the Company completed a sale of 328,669 shares of its common stock, and, to certain investors, pre-funded warrants to purchase 60,220 shares of its common stock and accompanying common stock warrants to purchase up to an aggregate of 388,920 shares of its common stock (the “June 2020 Offering”). Each share of common stock and pre-funded warrant to purchase one share of common stock was sold together with a common warrant to purchase one share of common stock. The shares of common stock and pre-funded warrants, and the accompanying common warrants, were issued separately and were immediately separable upon issuance. The pre-funded warrants were exercised in the third quarter of 2020. The common warrants were immediately exercisable at a price of $56.25 per share of common stock and will expire five years from the date of issuance. The common warrants provide the warrant holder with the right to participate in distributions on a 1-for-1 basis with common shareholders. No warrants associated with the June 2020 Offering were exercised during the years ended December 31, 2023 or 2022.
At Market Issuance Sales Agreements
In March 2021, the Company entered into an At Market Issuance Sales Agreement (the “2021 ATM Agreement”) with Oppenheimer & Co. Inc. (“Oppenheimer”) and William Blair & Company, L.L.C. as the Company’s sales agents (the “Agents”). The 2021 ATM Agreement was subsequently terminated effective December 22, 2023. Pursuant to the terms of the 2021 ATM Agreement, the Company could sell from time to time through the Agents shares of its common stock having an aggregate offering price of up to $50.0 million. Sales of shares were made by means of ordinary brokers’ transactions on The Nasdaq Capital Market at market prices or as otherwise agreed by the Company and the Agents. Under the terms of the 2021 ATM Agreement, the Company could also sell the shares from time to time to an Agent as principal for its own account at a price to be agreed upon at the time of sale. Any sale of the shares to an Agent as principal would have been sold pursuant to the terms of a separate placement notice between the Company and such Agent. During the year ended December 31, 2023, the Company sold 2,887,535 shares of common stock under the 2021 ATM Agreement at a weighted-average price of $2.34 per share, for aggregate net proceeds of $6.6 million, after giving effect to a 3% commission to the Agents. During the year ended December 31, 2022, the Company sold 354,381 shares of common stock under the 2021 ATM Agreement at a weighted-average price of $3.70 per share, for aggregate net proceeds of $1.3 million, after giving effect to a 3% commission to the Agents. As of the date the 2021 ATM Agreement was terminated, approximately $38.0 million of shares of common stock were remaining, but had not yet been sold by the Company under the 2021 ATM Agreement.
In April 2020, the Company entered into an At Market Issuance Sales Agreement (the “2020 ATM Agreement”) with Oppenheimer as the Company’s sales agent. The 2020 ATM Agreement was subsequently terminated effective December 22, 2023. Pursuant to the terms of the 2020 ATM Agreement, the Company could sell from time to time through Oppenheimer shares of its common stock having an aggregate offering price of up to $8.0 million. During the years ended December 31, 2023 and 2022, no sales of common stock under the 2020 ATM Agreement occurred. As of the date the 2020 ATM Agreement was terminated, approximately $2.6 million of shares of common stock were remaining, but had not yet been sold by the Company under the 2020 ATM Agreement.
Private Placement Offerings
In February 2020, the Company and Lincoln Park Capital Fund, LLC (“Lincoln Park”) entered into (i) a securities purchase agreement (the “Securities Purchase Agreement”); (ii) a purchase agreement (the “Purchase Agreement”); and (iii) a registration rights agreement. Pursuant to the Securities Purchase Agreement, Lincoln Park purchased, and the Company sold, (i) an aggregate of 21,111 shares of common stock (the “Common Shares”); (ii) a warrant to initially purchase an aggregate of up to 13,476 shares of common stock at an exercise price of $0.45 per share (the “Series A Warrant”); and (iii) a warrant to initially purchase an aggregate of up to 34,588 shares of common stock at an exercise price of $52.20 per share (the “Series B Warrant” and, together with the Series A Warrant, the “Warrants”). The Warrants provide the warrant holder with the right to
participate in distributions on a 1-for-1 basis with common shareholders. No Warrants associated with the Securities Purchase Agreement were exercised during the years ended December 31, 2023 or 2022.
Under the terms and subject to the conditions of the Purchase Agreement, the Company had the right, but not the obligation, to sell to Lincoln Park, and Lincoln Park was obligated to purchase, up to $28.0 million in the aggregate of shares of common stock. On September 1, 2023, the Purchase Agreement expired according to its terms. During the years ended December 31, 2023 and 2022, no sales of common stock under the Purchase Agreement occurred.
Preferred Stock
Under the Company’s Restated Certificate of Incorporation, the Company’s Board has the authority to issue up to 5,000,000 shares of preferred stock with a par value of $0.01 per share, at its discretion, in one or more classes or series and to fix the powers, preferences and rights, and the qualifications, limitations, or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption, and liquidation preferences, without further vote or action by the Company’s stockholders.
On May 25, 2022, the Company issued and sold one share of the Company’s preferred stock, which was designated as Series A Preferred Stock (the “Series A Preferred Stock”), for a nominal amount. During the time the Series A Preferred Stock was outstanding, it had 80,000,000 votes exclusively with respect to any proposal to amend the Company’s Restated Certificate of Incorporation to effect a reverse stock split of the Company’s common stock. The terms of the Series A Preferred Stock provided that it would be voted, without action by the holder, on any such proposal in the same proportion as shares of the Company’s common stock were voted. The Series A Preferred Stock otherwise had no voting rights except as otherwise required by the General Corporation Law of the State of Delaware. The Series A Preferred Stock was not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company and had no rights with respect to any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Series A Preferred Stock was not entitled to receive dividends of any kind. The Series A Preferred Stock was redeemed in whole on July 5, 2022 upon the effectiveness of the amendment to the Restated Certificate of Incorporation implementing the reverse stock split. As of December 31, 2023, there were no shares of preferred stock outstanding.
NOTE 7. STOCK-BASED COMPENSATION
Equity Incentive Plans
On December 27, 2023, the Company’s Board terminated the Fresh Tracks Therapeutics, Inc. 2020 Omnibus Long-Term Incentive Plan (the “2020 Plan”), the Amended and Restated 2009 Equity Incentive Plan of Brickell Biotech, Inc. (the “2009 Plan”), and the Amended and Restated Stock Incentive Plan of Vical Incorporated (the “Vical Plan”), and all outstanding options and unvested restricted stock units (“RSUs”) granted thereunder, effective December 28, 2023.
On April 20, 2020, the Company’s stockholders approved the 2020 Plan, which replaced, with respect to new award grants, the 2009 Plan and the Vical Plan (collectively, the “Prior Plans”) that were previously in effect. Following the approval of the 2020 Plan on April 20, 2020, no further awards were available to be issued under the Prior Plans but awards outstanding under those plans as of that date remained outstanding in accordance with their terms.
Because the exercise price of each of the outstanding stock options as of December 28, 2023 was above the closing price of the Company’s common stock on December 28, 2023, the Board cancelled and terminated the outstanding options as of December 28, 2023 without any consideration. With respect to the outstanding RSUs
on December 28, 2023, the Board decided to replace the value of the terminated RSUs with a right to a cash payment, subject to the same vesting schedule and payment date as the RSUs, with such cash payments valued by multiplying the number of RSUs held by each grantee by the closing price of the Company’s common stock on December 28, 2023.
Fair Value Assumptions
The Company accounts for share-based compensation expense for stock options granted to employees, members of its Board, and non-employees by estimating the fair value of each stock-based award on the date of grant using the Black-Scholes option pricing model. The Company recognizes share-based compensation expense on a straight-line basis over the vesting term. The Company applies an estimated forfeiture rate based on past history and makes revisions, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company considers the fair value of common stock to be equal to its current share price. If applicable, the current share price is adjusted to reflect material nonpublic information known to the Company but unavailable to market participants. The determination of the fair value of stock-based awards on the date of grant using an option-pricing model is affected by the value of the Company’s stock price, as well as assumptions regarding subjective variables. These variables include expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rate, and expected dividends.
Because the Company has a limited history of stock purchase and sale activity, the Company estimates expected volatility of the common stock by using the average share fluctuations of companies similar in size, operations, and life cycle. The expected term of stock options granted to employees, including members of the Board, is determined as the midpoint between the vesting date and the contractual end of the option grant. The expected term of all other stock options granted is based on the Company’s historical share option exercise experience, which approximates the midpoint between the vesting date and the contractual end of the option grant. The risk-free interest rates used in the valuation model are based on U.S. Treasury yield issues in effect at the time of grant for a period commensurate with the expected term of the grant. The Company does not anticipate paying any dividends in the foreseeable future and therefore uses an expected dividend yield of zero.
Stock Options
Stock options granted by the Company have an exercise price per share equal to the closing sales price of the common stock on the day prior to the date of grant and expire ten years from the date of grant. The vesting term of granted stock options is stated in each individual grant agreement, which is generally four years. During the year ended December 31, 2023, the Company did not grant any stock options. During the year ended December 31, 2022, the Company granted stock options with a weighted-average grant date fair value of $7.95 per share. The assumptions used to calculate the fair value of stock options granted are as follows, presented on a weighted-average basis:
| | | | | | | | | | | |
| Year Ended
December 31, |
| 2023 | | 2022 |
| | | |
| Expected term | N/A | | 6.0 years |
| Expected volatility | N/A | | 97.8% |
| Risk-free interest rate | N/A | | 3.1% |
| Expected dividend yield | N/A | | —% |
A summary of stock option activity under the Company’s incentive plans is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Shares | | Weighted-
Average
Exercise
Price | | Total
Intrinsic
Value | | Weighted-Average Remaining Contractual Life
(In Years) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Outstanding as of December 31, 2022 | 215,316 | | | $ | 84.10 | | | $ | — | | | 8.21 |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Forfeited | (67,876) | | | $ | 61.88 | | | | | |
| Expired | (3,452) | | | $ | 573.92 | | | | | |
| Cancelled | (143,988) | | | $ | 82.83 | | | | | |
| Outstanding as of December 31, 2023 | — | | | $ | — | | | $ | — | | | — |
| | | | | | | |
| | | | | | | |
As of December 31, 2023, the Company had no unrecognized share-based compensation expense related to stock options. The total estimated grant date fair value of stock options vested during the years ended December 31, 2023 and 2022 was $1.6 million and $2.3 million, respectively.
Restricted Stock Units
RSU activity during the year ended December 31, 2023 is shown below:
| | | | | | | | | | | | |
| Shares | | Weighted Average Grant Date Fair Value | |
| Unvested as of December 31, 2022 | — | | | $ | — | | |
| Granted | 141,250 | | | $ | 2.40 | | |
| Vested (1) | (110,000) | | | $ | 2.40 | | |
| Forfeited | (8,750) | | | $ | 2.40 | | |
| Repurchased (2) | (22,500) | | | $ | 2.40 | | |
| Unvested as of December 31, 2023 | — | | | $ | — | | |
____________
(1)The Company issued 66,831 shares of common stock in settlement of 110,000 vested RSUs, net of shares withheld for taxes.
(2)On December 28, 2023, there were 22,500 outstanding RSUs that were terminated and replaced with a right to a cash payment, subject to the same vesting schedule as the RSUs. In December 2023, a liability was established for the aggregate cash payment, and in January 2024, the Company paid an aggregate amount of $20 thousand to the RSU holders. The Company has no pattern of cash settling equity awards.
The total grant date fair value and the total vest date fair value of RSUs vested during the year ended December 31, 2023 were both approximately $0.3 million. As of December 31, 2023, the Company had no unrecognized share-based compensation expense related to RSU awards.
Employee Stock Purchase Plan
On April 19, 2021, the Company’s stockholders approved the Fresh Tracks Therapeutics, Inc. Employee Stock Purchase Plan (the “ESPP”), which had a first eligible purchase period commencing on July 1, 2021. The ESPP allowed qualified employees to purchase shares of the Company’s common stock at a price per share equal to 85% of the lower of: (i) the closing price of the Company’s common stock on the first trading day of the applicable purchase period or (ii) the closing price of the Company’s common stock on the last trading day of
the applicable purchase period. New six-month purchase periods began each January 1 and July 1. On December 14, 2023, the Company’s Board terminated the ESPP. The final purchase period under the ESPP began on January 1, 2023 and ended on June 30, 2023.
Stock-Based Compensation Expense
Total stock-based compensation expense reported in the consolidated statements of operations was allocated as follows (in thousands):
| | | | | | | | | | | | | | | |
| | | Year Ended
December 31, |
| | | | | 2023 | | 2022 |
| Research and development | | | | | $ | 468 | | | $ | 425 | |
| General and administrative | | | | | 1,451 | | | 1,731 | |
| Total stock-based compensation expense | | | | | $ | 1,919 | | | $ | 2,156 | |
Because all outstanding stock options were cancelled as of December 28, 2023, the Company recognized remaining compensation of $0.2 million during the year ended December 31, 2023 that would have been recognized in future periods had the stock options not been cancelled.
During the year ended December 31, 2023, the Company recognized $0.7 million of share-based compensation expense for the termination of certain executives. Employment agreements for these former executives included a service condition such that upon termination without cause, all unvested equity awards would accelerate and become fully vested as of the termination date.
NOTE 8. INCOME TAXES
During the years ended December 31, 2023 and 2022, the Company recorded no income tax benefits for the NOL incurred in each year, due to its uncertainty of realizing a benefit from those items.
A reconciliation of the U.S. federal statutory income tax rate to the Company’s effective income tax rate is as follows:
| | | | | | | | | | | |
| Year Ended
December 31, |
| 2023 | | 2022 |
| Federal statutory income tax rate | 21.00 | % | | 21.00 | % |
| State taxes, net of federal benefit | 1.55 | | | 2.88 | |
| | | |
| Research and development tax credits | — | | | 1.46 | |
| Permanent differences and other | (25.83) | | | (0.92) | |
| | | |
| Stock-based compensation | (3.28) | | | (0.46) | |
| | | |
| Change in deferred tax asset valuation allowance | 6.56 | | | (23.96) | |
| Effective income tax rate | — | % | | — | % |
Approximate deferred tax assets (liabilities) resulting from timing differences between financial and tax bases were associated with the following items (in thousands):
| | | | | | | | | | | |
| Year Ended
December 31, |
| 2023 | | 2022 |
| NOL carryforwards | $ | 106,071 | | | $ | 104,278 | |
| Research and development and other tax credits | 17,340 | | | 17,247 | |
| Depreciable assets | 3,387 | | | 4,812 | |
| | | |
| Capitalized research and development costs | 3,223 | | | 2,347 | |
| Intangible assets | 1,664 | | | 1,797 | |
| Stock-based compensation | — | | | 1,505 | |
| | | |
| Other | 9 | | | 82 | |
| Net deferred tax asset | 131,694 | | | 132,068 | |
| Less: valuation allowance | (131,694) | | | (132,068) | |
| Net deferred tax assets | $ | — | | | $ | — | |
As of December 31, 2023, the Company had deferred tax assets of $131.7 million. Due to uncertainties surrounding the Company’s ability to generate future taxable income to realize these assets, a full valuation allowance has been established to offset the net deferred tax asset.
Pursuant to Sections 382 and 383 of the Internal Revenue Code (“IRC”), annual use of the Company’s NOL and credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The most recent Section 382 analysis was completed through December 31, 2011 as a result of a previous ownership change on December 29, 2006, as determined per the provisions of Section 382 of the IRC as a result of various stock issuances used to finance the Company’s operations. Such ownership change resulted in annual limitations on the utilization of tax attributes, including NOL carryforwards and tax credits. A Section 382 analysis has not been conducted for the period between January 1, 2012 through December 31, 2023. As such, the Company cannot provide any assurance that a change in ownership within the meaning of the IRC has not occurred between those dates. If a change in ownership were to have occurred, additional NOL and tax credit carryforwards could be eliminated or restricted. If eliminated, the related asset would be removed from the deferred tax asset schedule with a corresponding reduction in the valuation allowance.
As of December 31, 2023 and 2022, the Company had available federal NOL carryforwards of approximately $432.7 million and $454.5 million, respectively. The NOLs generated after 2017, totaling $156.0 million, will carry forward indefinitely and be available to offset up to 80% of future taxable income each year. NOLs generated before 2018, totaling $276.7 million, will expire from 2024 through 2037.
In addition, the Company had federal research and development credits and orphan drug credit carryforwards of $23.5 million and $24.8 million as of December 31, 2023 and 2022, respectively, to reduce future federal income taxes, if any, which expire from 2024 through 2038. The Company also has available state NOL carryforwards of approximately $452.3 million and $444.4 million as of December 31, 2023 and 2022, respectively, which expire from 2028 to 2038.
All federal and state NOL and credit carryforwards listed above are reflected before the reduction for amounts effectively eliminated under Sections 382 and 383. Based upon statute, federal and state NOLs and credits are expected to expire as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Expiration Date: | Federal NOLs | | State NOLs | | Federal R&D Credit | | Federal Orphan Drug Credit | | State R&D Credit |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| 2024 | 25,032 | | | — | | | 213 | | | 663 | | | — | |
| 2025 | 27,190 | | | — | | | 455 | | | 507 | | | — | |
| 2026 | 21,858 | | | — | | | 451 | | | 302 | | | — | |
| 2027 and thereafter | 202,583 | | | 390,917 | | | 7,923 | | | 13,003 | | | — | |
| Indefinite | 156,032 | | | 61,353 | | | — | | | — | | | 9,763 | |
| Totals | $ | 432,695 | | | $ | 452,270 | | | $ | 9,042 | | | $ | 14,475 | | | $ | 9,763 | |
The Company has evaluated the positive and negative evidence bearing upon its ability to realize the deferred tax assets. Management has considered the Company’s history of cumulative net losses incurred since inception and its lack of commercialization of any products and has concluded that it is more likely than not that the Company will not realize the benefits of the deferred tax assets. Accordingly, a full valuation allowance has been established against the deferred tax assets as of December 31, 2023 and 2022. Management reevaluates the positive and negative evidence at each reporting period. The Company’s valuation allowance decreased by approximately $0.4 million for the year ended December 31, 2023. For the year ended December 31, 2022, the valuation allowance increased by $5.1 million.
The Company recognizes liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement. While the Company believes that it has appropriate support for the positions taken on its tax returns, the Company regularly assesses the potential outcome of examinations by tax authorities in determining the adequacy of its provision for income taxes.
The Company had previously acquired gross unrecognized tax benefits with a balance of $21.7 million as of December 31, 2023 and 2022, none of which would affect the effective tax rate, due to the Company’s full valuation allowance on its deferred tax assets. The Company does not anticipate any significant decreases in its unrecognized tax benefits over the next 12 months.
As of December 31, 2023, the Company’s U.S. federal and state tax returns remain subject to examination by tax authorities beginning with the tax year ended December 31, 2020. However, due to NOLs and credit carryforwards being generated and carried forward from prior tax years, substantially all tax years may also be subject to examination.
Effective for tax years beginning after December 31, 2021, Section 174 of the IRC requires that research and experimental expenses be capitalized and amortized. The amortization period is five years for domestic expenses and 15 years for foreign expenses. For the year ended December 31, 2023, the Company analyzed its expenses and determined that expenses of $5.0 million fell within the definition of Section 174. Accordingly, these expenditures were capitalized and amortized for tax purposes.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in the reports that we file under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the SEC, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosures. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the design and operation of our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) and 15d-15(e) promulgated under the Exchange Act, as of the end of the period covered by this Annual Report. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective and were operating at a reasonable assurance level as of December 31, 2023.
Management Report on Internal Controls over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of consolidated financial statements for external purposes in accordance with U.S. GAAP.
Management assessed our internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Management’s assessment included evaluation of elements such as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment.
Based on this assessment, management has concluded that our internal control over financial reporting was effective as of the end of the fiscal year to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external reporting purposes in accordance with U.S. GAAP. We reviewed the results of management’s assessment with our Board.
Inherent Limitations on Effectiveness of Controls
Our management, including the principal executive officer and principal financial officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well-designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected.
Changes in Internal Control over Financial Reporting
Management has determined that there were no changes in our internal control over financial reporting that occurred during the three months ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
During the three months ended December 31, 2023, none of our directors or officers (as defined in Rule 16a-1(f) of the Exchange Act) adopted or terminated any contract, instruction, or written plan for the purchase or sale of our securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) of the Exchange Act or any non-Rule 10b5-1 trading arrangement (as defined in the SEC’s rules).
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Our Director and Executive Officers
On September 18, 2023, our Board appointed Albert N. Marchio, II, the Company’s then-current Chief Financial Officer, as the Company’s new Chief Executive Officer, Chief Financial Officer, and Secretary, as well as a director and Chairman of the Board, in each case effective October 2, 2023. In addition, effective October 2, 2023, each of the prior members of the Board (Reginald L. Hardy, Gary A. Lyons, and Vijay B. Samant) resigned from the Board in conjunction with the Plan of Dissolution and not as a result of any disagreement on any matter relating to the operations, policies or practices of the Company. There are no family relationships among our director or executive officer.
Albert N. Marchio, II, Chairman of the Board, Chief Financial Officer, Chief Executive Officer
Mr. Marchio, age 71, a consultant with Danforth Advisors, currently serves as the Company’s Chairman of the Board, Chief Financial Officer, and Chief Financial Officer since September 2023 and as the Company’s Chief Financial Officer since December 2020. Mr. Marchio has been a consultant with Danforth Advisors since May 2019, providing financial consulting services on a project/interim basis for public (CytomX Therapeutics, Inc. (Nasdaq: CTMX)) and various private life sciences companies. Previously, Mr. Marchio served in various finance and accounting roles at Edge Therapeutics, Inc. (now known as PDS Biotechnology Corporation), a clinical-stage biopharmaceutical company, including Chief Accounting and Administrative Officer from October 2016 to November 2018, Interim Chief Financial Officer from March 2017 to October 2017, Chief Accounting and Operations Officer from March 2014 to October 2016, and Chief Financial Officer from December 2011 through March 2014. Mr. Marchio was a Managing Operating Partner with Three Fields Capital, a multi-strategy healthcare-focused investment firm, and provided consulting services to life science companies through Rockabye Valley Consulting from January 2009 to May 2013. Previously, Mr. Marchio served as the Executive Vice President, Chief Financial Officer of Informed Medical Communications from February 2008 to October 2009, and as the Vice President, Treasurer of MedPointe Pharmaceuticals from 2006 to January 2008. He began his career in life sciences as the Vice President, Treasurer of Alpharma, Inc. from 1992 to 2005. Mr. Marchio holds a B.A. in Economics from Muhlenberg College, an M.B.A. in Professional Accounting from Rutgers Graduate School of Business, and a Post-M.B.A. Certificate in Taxation from Bernard Baruch College of the City University of New York.
Audit Committee
On September 18, 2023, in connection with the approval of the Plan of Dissolution and the resignation of each of Messrs. Hardy, Lyons and Samant from the Board, the Board dissolved the Company’s Audit Committee, effective October 2, 2023.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics (“Code of Ethics”) and a whistleblower policy for anonymous reporting applicable to all of our officers, directors, and employees, which can be accessed in the Investors section on our website at www.frtx.com. If we make any substantive amendments to our Code of Ethics and/or whistleblower policy or grant any waiver from a provision of the Code of Ethics to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires the Company’s officers and directors, and persons who beneficially own more than ten percent of a registered class of the Company’s equity securities, to file reports of ownership and changes in ownership on Forms 3, 4 and 5 with the SEC.
To our knowledge, based solely on a review of the Section 16(a) reports filed electronically with the SEC during the year ended December 31, 2023 and written representations from applicable Company officers and directors that no other reports were required, all Section 16(a) filing requirements were timely met with respect to the year ended December 31, 2023, except for the following:
•Form 4 filed on December 11, 2023, reporting the accelerated vesting of restricted stock units (“RSUs”), and the associated shares withheld upon vesting, for the Company’s former executive officer Mr. Chadha;
•Form 3 filed on December 1, 2023, reporting initial common stock and equity awards held by Mr. Fox-Collis; and
•Form 4 filed on January 9, 2024, reporting the replacement of previously awarded RSUs with a corresponding cash payment for both Mr. Fox-Collis and Mr. Marchio.
ITEM 11. EXECUTIVE COMPENSATION
Executive Compensation
The primary objectives of the Compensation Committee of our Board, and subsequent to the dissolution of the Compensation Committee effective October 2, 2023, the Board, with respect to executive compensation have been to retain and motivate its executives in a year of strategic change for the Company associated with evaluating potential strategic alternatives, approving the Plan of Dissolution, and facilitating the wind down of the Company. The Compensation Committee and Board have maintained compensation plans that tie a substantial portion of executives’ overall compensation to these priorities.
This section discusses the material components of the executive compensation program offered to our executives, and in particular to our named executive officers for 2023, who were:
•Albert N. Marchio, II, Current Chief Executive Officer and Chief Financial Officer;
•Robert B. Brown, Former Chief Executive Officer;
•Andrew D. Sklawer, Former President, Chief Executive Officer, and Chief Operating Officer;
•David R. McAvoy, Former General Counsel, Chief Compliance Officer, and Secretary; and
•Deepak Chadha, Former Chief Operating Officer and Chief Research and Development Officer.
Changes in Executive Officer Roles
On September 19, 2023, we announced the Plan of Dissolution and our intent to discontinue all clinical and preclinical development programs and reduce our workforce. In connection with the Plan of Dissolution, effective October 2, 2023, we discontinued all clinical and preclinical development programs and terminated most of our employees, except for certain employees, consultants, and advisors who will supervise or facilitate the dissolution and wind down of the Company.
Mr. Marchio was appointed as the Company’s Chief Executive Officer, Chief Financial Officer, and Secretary, as well as a director and Chairman of the Board, in each case effective October 2, 2023. Mr. Marchio succeeded Mr. Sklawer, who since January 31, 2023, was appointed and served as the Company’s President and Chief Executive Officer, and prior to that date, he served as President and Chief Operating Officer. Mr. Sklawer was terminated without cause effective October 2, 2023 (the “Separation Date”). Mr. Sklawer succeeded Mr. Brown,
the former Chief Executive Officer of the Company, who notified the Company in January 2023 of his decision to retire and resign, effective January 31, 2023. Mr. Brown resigned as a member of the Board on July 24, 2023.
Effective September 20, 2022, the Board appointed Mr. Chadha, the Company’s then-Chief Research and Development Officer, to the additional position of Chief Operating Officer, resulting in Mr. Chadha holding both positions until September 2023, when Mr. Chadha was terminated without cause from the Company.
Mr. McAvoy joined the Company in 2019 and served since then as its General Counsel and Chief Compliance Officer. He also was appointed Secretary in May 2022. Mr. McAvoy was terminated without cause from the Company in September 2023.
Determination of Executive Compensation
All compensation decisions affecting our executive officers, including the named executive officers, are solely determined by the Compensation Committee or, after the dissolution of the Compensation Committee, the Board.
Historically, after performing individual evaluations, our Chief Executive Officer would submit recommendations for approval to the Compensation Committee for salary increases, annual cash incentive bonuses, and stock-based awards for the other executives. In the case of the Chief Executive Officer, his individual performance evaluation was conducted by the Compensation Committee, which solely determined his base salary, annual cash incentive bonus, and stock-based awards. The Compensation Committee retained ultimate discretion as to whether any salary increases, annual cash incentive bonuses, stock-based awards, or retention bonuses would be awarded for any year, including whether to accept or vary from the Chief Executive Officer’s recommendations for other executives. Subsequent to the dissolution of the Compensation Committee, the Board makes all compensation decisions in its sole discretion.
In addition to corporate and individual goal achievement, the Compensation Committee and Board also considered the following factors in determining any of our executive’s compensation package(s):
•the executive’s role and performance within the Company and the compensation data for similar persons in peer-group companies and third-party benchmarked compensation survey data;
•the demand for executives with the executive’s specific expertise and experience;
•a comparison to other executives within the Company having similar levels of expertise and experience; and
•uniqueness of the executive’s industry skills.
Compensation Components
The components of our compensation package are as follows:
Base Salary
Base salaries for our executives are established based on the scope of their responsibilities and their prior relevant background, training, and experience, taking into account competitive market compensation paid by the companies represented in our peer group for similar positions and the overall market demand for such executives at the time of hire. An executive’s base salary is also evaluated together with other components of the executive’s compensation to ensure that the executive’s total compensation is in line with our overall compensation philosophy.
Base salaries are reviewed annually as part of our annual performance program and increased for merit reasons, based on the executive’s success in meeting or exceeding individual performance objectives and an assessment of whether significant corporate goals were achieved. If necessary, we also realign base salaries with market levels for the same positions in the companies in our peer group if we identify significant market changes in our data analysis. Additionally, the Compensation Committee or, after the dissolution of the Compensation Committee, the Board adjusts base salaries as warranted throughout the year for promotions or other changes in the scope or breadth of an executive’s role or responsibilities.
Annual Cash Incentives
Our compensation program includes eligibility for an annual incentive cash bonus in the case of all executives and certain non-executive employees. For our executives, the amount of the cash bonus depends on the level of achievement of the stated corporate performance goals, with a target bonus set as a percentage of base salary.
Long-Term Incentives
Although the Company’s equity compensation plans were terminated in December 2023 in conjunction with the Company’s Plan of Dissolution, prior to this, we believed that long-term performance was achieved through an ownership culture to encourage long-term participation by our executives through equity-based awards. Our 2020 Omnibus Long-Term Incentive Plan (the “2020 Plan”) previously allowed the grant to executives of stock options, RSUs, and other equity-based awards. Historically, we typically have made an initial equity award of stock options to new employees and annual stock-based grants as part of our overall compensation program. The cumulative amount of stock options granted as part of our annual performance program has been approved by the Compensation Committee. All equity-based awards granted to executives were approved by our Compensation Committee or our Board.
Retirement Plan
We have established a 401(k) retirement savings plan that allows eligible employees to defer a portion of their compensation, within limits prescribed by the Internal Revenue Code (the “Code”), on a pre-tax or after-tax basis through contributions to the plan. Our named executive officers are eligible to participate in the 401(k) plan on the same general terms as other full-time employees. Effective January 1, 2022, the Company matches 100% on the first 3% of an employee’s contribution to the 401(k) Plan and matches 50% on the next 2% of an employee’s contribution to the 401(k) Plan.
Employee Stock Purchase Plan
Although the Company’s equity compensation plans were terminated in December 2023 in conjunction with the Company’s Plan of Dissolution, prior to this, the Company maintained an Employee Stock Purchase Plan (the “ESPP”). The ESPP permitted employees to acquire shares of our common stock through periodic payroll deductions during purchase periods of six months each. The purchase price of our common stock acquired on each purchase date under the ESPP generally was equal to 85% of the lesser of the fair market value of our common stock on (i) the first trading day of the purchase period or (ii) the last trading day of the purchase period.
Other Compensation
We maintain broad-based benefits and perquisites that are offered to all employees, including health insurance, life and disability insurance, and dental insurance. In particular circumstances, we may also utilize cash signing bonuses when certain executives join us, or provide retention bonuses in cash or equity as circumstances may warrant. Whether a signing (or retention) bonus is paid, and the amount thereof, is determined on a case-by-case basis under the specific circumstances. For example, we will consider paying a signing bonus to compensate for
amounts forfeited by an executive upon terminating prior employment, to assist with relocation expenses, and/or to create additional incentive for an executive to join (or stay with) our Company in a position where there is high market demand. Additionally, we have previously provided, as part of taxable compensation, payments of $3,000 per month for separate corporate apartments to a former Chief Executive Officer and former General Counsel, as they resided in different states from the Company’s headquarters.
Acceleration of Vesting of Equity-Based Awards
In addition to the severance provisions contained in the employment agreements with our former Chief Executive Officers and our other eligible executives, provisions of the 2020 Plan allowed our Board to grant stock-based awards to employees and executives that provided for the acceleration of vesting in the event of a “change in control” (as defined in the 2020 Plan). Our previously outstanding equity-based awards included provisions that accelerated vesting of such awards in the event of (i) a change in control in which the surviving or successor entity would not continue, assume, or replace the awards; and (ii) a termination of employment without cause or by the employee for good reason within 24 months after a change in control in which the surviving or successor entity would continue, assume, or replace the award. These provisions were designed to promote stability during any change of control and would enable our executives to focus on corporate objectives during a change of control, even if their employment may be subsequently terminated.
Prohibition on Pledging and Hedging
Under the terms of our insider trading policy, our executive officers and directors are prohibited from engaging in hedging or similar transactions designed to decrease the risks associated with holding our stock, including short sales and transactions in put or call options or derivative transactions (including but not limited to forward sale contracts, zero-cost collars or other hedging or monetization transactions).
Our insider trading policy also prohibits our executive officers and directors from pledging our stock as collateral for loans.
Tax Implications
As part of its role, the Compensation Committee or Board reviews and considers the deductibility of executive compensation under Section 162(m) of the Code, which provides that we may not deduct compensation of more than $1,000,000 that is paid to certain individuals. We believe that compensation paid under the management incentive plans is generally fully deductible for federal income tax purposes. However, in certain situations, the Compensation Committee or Board may approve compensation that will not meet these requirements in order to ensure competitive levels of total compensation for our executives.
Accounting for Stock-Based Compensation
We have accounted for stock-based compensation in accordance with the requirements of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 718. The Compensation Committee and Board consider the accounting impact of equity-based compensation when developing its compensation strategy.
The Role of Stockholder Say-on-Pay Votes
Historically, we provided our stockholders with the opportunity to cast an advisory (non-binding) vote on executive compensation through a “say-on-pay” proposal every three years, which was the preference expressed by stockholders at our 2020 Annual Meeting. Because this vote is advisory, it was not binding on the Board or the Compensation Committee. However, the Board and the Compensation Committee value the opinion of our
stockholders and will consider their concerns when making future decisions regarding the structure and implementation of our executive compensation program.
At our 2020 Annual Meeting, approximately 84% of votes cast were voted in favor of the say-on-pay proposal.
Compensation Decisions for 2023
During the majority of 2023, our Board and executive management team were conducting a comprehensive process to explore and evaluate strategic alternatives with the goal of maximizing stockholder value. Potential alternatives that were under evaluation included, but were not limited to, a financing, a merger or reverse merger, the sale of all or part of the Company, licensing of assets, a business combination, and/or other strategic transactions or series of related transactions involving the Company. Therefore, compensation decisions were prioritized around these initiatives and focused on retaining human capital resources for this process and its outcome.
Base Salary
Historically, the Compensation Committee reviewed and approved salaries for the Chief Executive Officer and each of the other executives on an annual basis or at other times as necessary to accommodate the hiring of new employees, a change in responsibilities, promotions, or other considerations. The Chief Executive Officer provides recommendations to the Compensation Committee for each of the executives other than himself. Recommended base salaries were reviewed and set based on a number of factors, including but not limited to job responsibilities, individual industry experience, position, changes in responsibilities, individual performance, our overall performance, and peer-group data for comparable positions. No predetermined weight is given to any of the above factors. Subsequent to the dissolution of the Compensation Committee, the Board makes all compensation decisions in its sole discretion.
In January 2023, unless otherwise noted, the Compensation Committee approved the following increases in base salary, effective January 1, 2023:
| | | | | | | | | | | | | | |
| Name | | Base Salary | | Percentage Increase from 2022 |
| Albert N. Marchio, II (1) | | N/A | | N/A |
| Robert B. Brown | | $ | 530,303 | | | 5.0 | % |
| Andrew D. Sklawer | | $ | 441,000 | | | 5.0 | % |
| Deepak Chadha | | $ | 415,000 | | | 9.6 | % |
David R. McAvoy | | $ | 350,000 | | | 16.0 | % |
_________________
(1)Mr. Marchio has served as a consultant of the Company through Danforth Advisors since December 2020. The Company makes payment for services provided by Mr. Marchio pursuant to a consulting agreement by and between the Company and Danforth Advisors LLC, which provides for compensation for services provided at a rate of $350 per hour, as well as reimbursement of Mr. Marchio’s covered commuting expenses to the Company’s Boulder, Colorado location and any other such necessary business expenses.
Annual Cash Incentives and Bonuses
The following table sets forth the 2023 target annual cash incentive bonus for each named executive officer as a total dollar amount and percentage of base salary, as set by the Compensation Committee. However, compensation decisions were prioritized around the Company’s comprehensive process to explore and evaluate strategic alternatives for the Company and its ultimate outcome, no performance objectives were approved by
the Compensation Committee, and no objective targets were determined to be achieved for 2023. Thus, no incentive bonuses associated with 2023 were paid.
| | | | | | | | | | | | | | | | | |
| Name | | | | Target Bonus
Percentage Amount | | Target Bonus
Percentage | |
| Albert N. Marchio, II (1) | | | | N/A | | N/A | |
| Robert B. Brown | | | | $ | 530,303 | | | 50 | % | |
| Andrew D. Sklawer | | | | $ | 441,000 | | | 50 | % | |
| Deepak Chadha | | | | $ | 415,000 | | | 40 | % | |
| David R. McAvoy | | | | $ | 350,000 | | | 40 | % | |
Long-Term Equity-Based Incentives
Although our equity compensation plan terminated in December 2023, we historically granted long-term equity-based incentive awards under the 2020 Plan, and prior to the approval of the 2020 Plan, the Amended and Restated 2009 Equity Incentive Plan of Brickell Biotech, Inc., and the Amended and Restated Stock Incentive Plan of Vical Incorporated, at the discretion of the Compensation Committee. The Compensation Committee relied in large part on the recommendation of our Chief Executive Officer in determining the value of equity-based incentive awards to be granted to our other executives. The Compensation Committee also considered job responsibilities, individual industry experience, position, changes in responsibilities, individual performance, our overall performance and peer-group data for comparable positions. With the exception of new employees and promotions, equity-based incentive awards were typically granted on an annual basis during the first quarter of the fiscal year after the Compensation Committee had reviewed financial projections for the fiscal year prepared by management at the Compensation Committee’s request.
In 2023, the Compensation Committee granted the following number of RSUs to each of the named executive officers with an effective grant date of January 24, 2023:
| | | | | | | | | |
| Name | | Number of RSUs (1) | |
Albert N. Marchio, II (2) | | 1,250 | |
Robert B. Brown (3) | | — | |
Andrew D. Sklawer (4) | | 55,000 | |
Deepak Chadha (5) | | 35,000 | |
David R. McAvoy (4) | | 20,000 | |
_________________
(1)These RSUs were granted on January 24, 2023, with each original award vesting in full on January 24, 2024 and to be paid in the form of shares of the Company’s common stock, subject to continued employment through the vesting date.
(2)On December 28, 2023, the Board terminated all of the Company’s equity incentive plans, and Mr. Marchio’s RSUs were replaced with a right to a cash payment on the vest date of $1,138, which was paid to Mr. Marchio in January 2024.
(3)Because Mr. Brown notified the Company on January 23, 2023 of his decision to retire and resign, effective January 31, 2023, he was not eligible to receive an RSU award.
(4)Mr. Sklawer’s and Mr. McAvoy’s RSU awards were subject to accelerated vesting upon their termination from the Company on October 2, 2023.
(5)Mr. Chadha’s RSU award was subject to accelerated vesting upon his termination from the Company on September 1, 2023.
Summary Compensation Table
The following table presents information regarding compensation earned by or awarded to our named executive officers during the years ended December 31, 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and Principal Position(1) | | Year | | Salary
($) | | | Bonus ($)(2) | | Stock Awards ($)(3) | | | Option Awards ($)(3) | | Non-Equity Incentive Plan Compensation ($)(4) | | All Other Compensation ($)(5)(6)(7) | | Total
($) |
| | | | | | | | | | | | | | | | | | |
| Albert N. Marchio, II, Current Chief Executive Officer and Chief Financial Officer | | 2023 | | — | | | — | | 3,000 | | | — | | — | | 251,834 | | 254,834 |
| | | | | | | | | | | | | | | | | |
| Robert B. Brown, Former Chief Executive Officer | | 2023 | | 44,192 | | | — | | — | | | — | | — | | 237,996 | | 282,188 |
| 2022 | | 505,050 | | | — | | — | | | 205,629 | | 184,343 | | 36,000 | | 931,022 |
| Andrew D. Sklawer, Former President, Chief Executive Officer and Chief Operating Officer | | 2023 | | 333,200 | | | 88,200 | | 132,000 | | | — | | — | | 616,150 | | 1,169,550 |
| 2022 | | 420,000 | | | — | | — | | | 84,121 | | 122,640 | | — | | 626,761 |
| Deepak Chadha, Former Chief Operating Officer and Chief Research and Development Officer | | 2023 | | 277,820 | | | 83,000 | | 84,000 | | | — | | — | | 460,490 | | 905,310 |
| 2022 | | 378,530 | | | — | | — | | | 60,754 | | 110,531 | | — | | 549,815 |
| David R. McAvoy, Former General Counsel, Chief Compliance Officer, and Secretary | | 2023 | | 264,444 | | | 70,000 | | 48,000 | | | — | | — | | 365,652 | | 748,096 |
| | | | | | | | | | | | | | | | | |
_________________
(1)Mr. Marchio served as the Company’s Chief Financial Officer during 2022 and 2023 and was also appointed as the Company’s Chief Executive Officer and Secretary, as well as a director and Chairman of the Board, in each case effective as of October 2, 2023. Mr. Marchio succeeded Mr. Sklawer, who since January 31, 2023, was appointed and served as the Company’s President and Chief Executive Officer, and prior to that date, he served as President and Chief Operating Officer. Mr. Sklawer was terminated without cause effective on the Separation Date. Mr. Sklawer succeeded Mr. Brown, the former Chief Executive Officer of the Company, who notified the Company in January 2023 of his decision to retire and resign, effective January 31, 2023.
Effective September 20, 2022, the Board appointed Mr. Chadha, the Company’s then-Chief Research and Development Officer, to the additional position of Chief Operating Officer, resulting in Mr. Chadha holding both positions until September 1, 2023, at which date Mr. Chadha was terminated without cause from the Company.
Mr. McAvoy served as the Company’s General Counsel and Chief Compliance Officer during 2022 until September 2023, when Mr. McAvoy was terminated without cause from the Company.
(2)Relates to retention bonuses that were awarded in 2023, each of which was paid in two equal installments if the applicable executive remained employed by the Company or had not provided notice of intent to resign on and as of June 30, 2023 and December 31, 2023.
(3)These amounts represent the grant date fair value of equity-based awards granted by the Company during the years presented, determined in accordance with FASB ASC Topic 718. For a detailed discussion of our grant date fair value calculation methodology, including assumptions and estimates inherent therein, please refer to Note 7 to the consolidated financial statements contained within this Annual Report.
(4)Consists of cash bonus payments pursuant to pre-established performance objectives.
(5)Includes temporary living expenses provided to Mr. Brown pursuant to his employment or separation agreements in effect during 2022 and 2023 of $5,400 and $36,000, respectively. See “Employment Agreements” section below.
(6)Includes severance expenses and amounts paid for previously accrued but unused time off pursuant to employment agreements or separation agreements in effect during 2022 and 2023. Severance payments were paid in 2023 and 2024 to the following executive officers in the following amounts: Mr. Brown — $21,334; Mr. Sklawer — $441,000; Mr. Chadha — $415,000; and Mr. McAvoy — $262,500. Amounts for previously accrued but unused time off were paid in 2023 and 2024 to the following executive officers in the following amounts: Mr. Brown — $94,843; Mr. Sklawer — $165,375; Mr. Chadha — $45,490; and Mr. McAvoy — $39,038. See “Employment Agreements” section below.
(7)Includes payments for consulting services provided in 2023 by the following executive officers in the following amounts: Mr. Marchio — $251,834; Mr. Brown — $116,419; Mr. Sklawer — $9,775; and Mr. McAvoy — $64,114. See “Employment Agreements” section below for additional details of the consulting agreements.
Outstanding Equity Awards at Year-End
The Company’s equity compensation plans were terminated in December 2023 in conjunction with the Company’s Plan of Dissolution. Therefore, as of December 31, 2023, the Company’s named executive officers had no outstanding stock-based awards under any of the company’s prior equity compensation plans.
Employment Agreements
The Company has entered into employment agreements with each of its named executive officers as described below.
Albert N. Marchio, II
Mr. Marchio provides services on a part-time basis pursuant to a Consulting Agreement between the Company and Danforth Advisors, LLC (Mr. Marchio’s employer), effective December 1, 2020 (the “Marchio Consulting Agreement”). The term of the Marchio Consulting Agreement will continue until such time as either party gives written notice of termination. The Marchio Consulting Agreement provides for compensation for services provided at a rate of $350 per hour, as well as reimbursement of Mr. Marchio’s covered commuting expenses to our Boulder, Colorado location and any other such necessary business expenses.
Robert B. Brown
Employment Agreement
Under the terms of the employment agreement entered into between the Company and Robert B. Brown, Mr. Brown was entitled to an annual base salary of $450,000, subject to adjustment upon annual review by the Board, and was eligible for our benefit programs, vacation benefits, and medical benefits. In addition, Mr. Brown was entitled to a performance bonus of up to 50% of his base salary. Under the employment agreement, Mr. Brown was also eligible for reimbursement of relocation assistance of up to $3,000 a month for living expenses for 36 months (unless subsequently extended), along with up to $75,000 of one-time relocation expenses.
The agreement provided that upon written notice, either party could have terminated the employment arrangement with or without cause, but 15 days’ written notice was required if the agreement was terminated by Mr. Brown. In addition, the agreement provided that if we terminated Mr. Brown’s employment without cause or Mr. Brown terminated his employment for good reason, Mr. Brown would be eligible to receive:
•any unpaid base salary through the effective date of termination;
•any accrued but unpaid vacation;
•any accrued and/or pro-rated but unpaid incentive compensation;
•base salary for a period of 12 months paid in a lump sum; and
•continuation of health benefits under COBRA for 12 months.
The agreement further provided that if we terminated Mr. Brown’s employment without cause or Mr. Brown terminated his employment for good reason within 12 months following a change in control, Mr. Brown would have been eligible to receive:
•any accrued but unpaid personal days;
•fully accelerated vesting of all outstanding unvested options or other equity instruments;
•base salary for a period of 12 months in the form of salary continuation; and
•continuation of health benefits under COBRA for 12 months.
Transition and Release Agreement
Mr. Brown notified the Company in January 2023 of his decision to retire and resign as Chief Executive Officer, effective January 31, 2023. In connection with Mr. Brown’s resignation, on February 1, 2023, we and Mr. Brown entered into a transition and release agreement. Pursuant to the agreement, Mr. Brown received (i) a 2022 performance bonus of $184,343, (ii) a lump sum of $21,334 to reimburse certain future medical, vision, and dental insurance expenses for Mr. Brown and his spouse as part of his transition, (iii) $3,000 to mitigate the cost of terminating Mr. Brown’s apartment lease in Boulder, Colorado, and (iv) a lump sum of $94,843 as payment for previously accrued but unused paid time off as a Company employee. The transition and release agreement also provided that Mr. Brown will not receive any severance benefits pursuant to his former employment agreement, which terminated at the same time as his employment termination and included a release of claims in favor of the Company and customary confidentiality and non-disparagement provisions.
Consulting Agreement
In connection with Mr. Brown’s resignation as Chief Executive Officer, we and Dancing Bear Consulting, LLC, a limited liability company owned by Mr. Brown, entered into a consulting agreement, which became effective as of February 1, 2023, under which Mr. Brown will personally provide consulting and advisory services to the Company. The initial term of the consulting agreement is one year, subject to automatic renewal for additional one-year terms unless either party terminates. The consulting agreement provides for compensation at a fixed rate of $10,000 per month, as well as reimbursement of Mr. Brown’s related business expenses. Mr. Brown will provide consulting and advisory services as requested by the Company. If the consulting agreement had been terminated (i) without cause by the Company or (ii) by Mr. Brown for cause or in the event of our bankruptcy or insolvency, we would have been obligated to pay the remaining compensation that would have been payable
during the initial one-year term. The consulting agreement was terminated by the Company effective January 31, 2024.
Andrew D. Sklawer
Employment Agreement
On February 21, 2023, we entered into an amended and restated employment agreement with Mr. Sklawer, pursuant to which Mr. Sklawer’s annual base salary was $441,000, retroactive to January 1, 2023, subject to increase from time to time, and he was eligible to receive (i) an annual target performance bonus of 50% of his base salary, (ii) equity awards, and (iii) health insurance, retirement, and other benefits.
Upon written notice, either party could have terminated the agreement with or without cause, but 15-days’ written notice was required if the termination was by Mr. Sklawer. Upon Mr. Sklawer’s termination by the Company without cause or Mr. Sklawer’s termination of the agreement for good reason, and subject to his execution of a general release of claims in favor of the Company and its employees, officers and directors, Mr. Sklawer was entitled to receive severance payments equal to (i) 12 months of base salary (18 months if the termination had been within 12 months following a change in control of the Company) and (ii) the cost of health insurance for him and his eligible dependents for a period of 12 months (18 months if the termination had been within 12 months following a change in control of the Company). If Mr. Sklawer’s employment had been terminated by the Company without cause or by him for good reason within 12 months following a change in control of the Company, Mr. Sklawer also would have received an amount equal to 150% of his target performance bonus for the year in which the termination occurred. In addition, upon the termination of Mr. Sklawer’s employment for any reason other than by the Company for cause or due to his disability, all unvested equity awards fully vested, and an exercise period of three years from that accelerated vesting date applied. Subject to certain exceptions, the agreement also prohibited Mr. Sklawer from soliciting our current or former employees and actual or targeted clients and customers during the term of his employment and for one year following his date of termination.
Separation and Release Agreement
We terminated Mr. Sklawer without cause, effective on the Separation Date. In connection with Mr. Sklawer’s separation from the Company, on October 3, 2023, the Company and Mr. Sklawer entered into a separation and release agreement. Pursuant to the separation and release agreement, Mr. Sklawer received a lump sum of (i) $441,000 in severance, which is an amount equal to 12 months of Mr. Sklawer’s base salary in effect as of the Separation Date and (ii) $44,100 in accordance with that certain employee retention bonus agreement, dated as of February 21, 2023, between the Company and Mr. Sklawer. In addition, Mr. Sklawer was paid a lump sum of $165,375 for previously accrued but unused paid time off as a Company employee, and we paid for 12 months of Mr. Sklawer’s health care premiums; however, if we can no longer provide group health insurance for the full 12-month period, we will make a lump sum payment to Mr. Sklawer for the remaining premiums, grossed up by 35% to minimize the impact of any applicable taxes. Finally, all of Mr. Sklawer’s outstanding and unvested equity awards vested in full as of the Separation Date, and an exercise period of three years from that accelerated vesting date applied.
Consulting Agreement
In connection with Mr. Sklawer’s separation from the Company, we and Yonder Partners, LLC, a limited liability company owned by Mr. Sklawer, also entered into a consulting agreement on October 3, 2023, under which Mr. Sklawer will personally provide consulting and advisory services to the Company. The term of the consulting agreement continues until terminated, which either party may do (i) with cause upon 30 calendar days’ prior written notice or (ii) without cause upon 45 calendar days’ prior written notice. The consulting
agreement provides for compensation at a fixed rate of $425 per hour, as well as reimbursement of Mr. Sklawer’s related business expenses.
David R. McAvoy
Employment Agreement
On February 21, 2023, we entered into an amended and restated employment agreement with Mr. McAvoy, pursuant to which Mr. McAvoy’s annual base salary was $350,000, retroactive to January 1, 2023, subject to increase from time to time, and he was eligible to receive (i) an annual target performance bonus of 40% of his base salary, (ii) equity awards, and (iii) health insurance, retirement, and other benefits.
Upon written notice, either party could have terminated the agreement with or without cause, but 15-days’ written notice was required if the termination was by Mr. McAvoy. Upon Mr. McAvoy’s termination by the Company without cause or Mr. McAvoy’s termination of the agreement for good reason, and subject to his execution of a general release of claims in favor of the Company and its employees, officers and directors, Mr. McAvoy was entitled to receive severance payments equal to (i) nine months of base salary (12 months if the termination had been within 12 months following a change in control of the Company) and (ii) the cost of health insurance for him and his eligible dependents for a period of nine months (12 months if the termination had been within 12 months following a change in control of the Company). If Mr. McAvoy’s employment had been terminated by the Company without cause or by him for good reason within 12 months following a change in control of the Company, Mr. McAvoy also would have received an amount equal to 100% of his target performance bonus for the year in which the termination occurred. In addition, upon the termination of Mr. McAvoy’s employment for any reason other than by the Company for cause or due to his disability, all unvested equity awards fully vested, and an exercise period of three years from that accelerated vesting date applied. Subject to certain exceptions, the agreement also prohibited Mr. McAvoy from soliciting our current or former employees and actual or targeted clients and customers during the term of his employment and for one year following his date of termination.
Separation and Release Agreement
We terminated Mr. McAvoy without cause, effective on the Separation Date. In connection with Mr. McAvoy’s separation from the Company, on October 3, 2023, the Company and Mr. McAvoy entered into a separation and release agreement. Pursuant to the separation and release agreement, Mr. McAvoy received or is entitled to receive, (i) $262,500 in severance, which is an amount equal to nine months of Mr. McAvoy’s base salary in effect as of the Separation Date and (ii) $35,000 in accordance with that certain employee retention bonus agreement, dated as of February 21, 2023, between the Company and Mr. McAvoy. In addition, Mr. McAvoy was paid a lump sum of $39,038 for previously accrued but unused paid time off as a Company employee, and we will pay for nine months of Mr. McAvoy’s health care premiums; however, if we can no longer provide group health insurance for the full nine-month period, we will make a lump sum payment to Mr. McAvoy for the remaining premiums, grossed up by 35% to minimize the impact of any applicable taxes. Finally, all of Mr. McAvoy’s outstanding and unvested equity awards vested in full as of the Separation Date, and an exercise period of three years from that accelerated vesting date applied.
Consulting Agreement
In connection with Mr. McAvoy’s separation from the Company, we and McAvoy Law LLC, a limited liability company owned by Mr. McAvoy, also entered into a consulting agreement on October 3, 2023, under which Mr. McAvoy will personally provide consulting and advisory services to the Company. The term of the consulting agreement continues until terminated, which either party may do (i) with cause upon 30 calendar days’ prior written notice or (ii) without cause upon 45 calendar days’ prior written notice. The consulting
agreement provides for compensation at a fixed rate of $425 per hour, as well as reimbursement of Mr. McAvoy’s related business expenses.
Deepak Chadha
Employment Agreement
On February 21, 2023, we entered into an amended and restated employment agreement with Mr. Chadha, pursuant to which Mr. Chadha’s annual base salary was $415,000, retroactive to January 1, 2023, subject to increase from time to time, and he was eligible to receive (i) an annual target performance bonus of 40% of his base salary, (ii) equity awards, and (iii) health insurance, retirement, and other benefits.
Upon written notice, either party could have terminated the agreement with or without cause, but 15-days’ written notice was required if the termination was by Mr. Chadha. Upon Mr. Chadha’s termination by the Company without cause or Mr. Chadha’s termination of the agreement for good reason, and subject to his execution of a general release of claims in favor of the Company and its employees, officers and directors, Mr. Chadha was entitled to receive severance payments equal to (i) 12 months of base salary and (ii) the cost of health insurance for him and his eligible dependents for a period of 12 months. If Mr. Chadha’s employment had been terminated by the Company without cause or by him for good reason within 12 months following a change in control of the Company, Mr. Chadha also would have received an amount equal to 100% of his target performance bonus for the year in which the termination occurred. In addition, upon the termination of Mr. Chadha’s employment for any reason other than by the Company for cause or due to his disability, all unvested equity awards fully vested, and an exercise period of three years from that accelerated vesting date applied. Subject to certain exceptions, the agreement also prohibited Mr. Chadha from soliciting our current or former employees and actual or targeted clients and customers during the term of his employment and for one year following his date of termination.
Separation and Release Agreement
We terminated Mr. Chadha without cause, effective on September 1, 2023. In connection with Mr. Chadha’s separation from the Company, on October 6, 2023, the Company and Mr. Chadha entered into a separation and release agreement. Pursuant to the separation and release agreement, Mr. Chadha received (i) $415,000 in severance, which is an amount equal to 12 months of Mr. Chadha’s base salary in effect as of September 1, 2023 and (ii) $41,500 in accordance with that certain employee retention bonus agreement, dated as of February 21, 2023, between the Company and Mr. Chadha. In addition, Mr. Chadha was paid a lump sum of $45,490 for previously accrued but unused paid time off as a Company employee, and we will pay for 12 months of Mr. Chadha’s health care premiums; however, if we can no longer provide group health insurance for the full nine-month period, we will make a lump sum payment to Mr. Chadha for the remaining premiums, grossed up by 35% to minimize the impact of any applicable taxes. Finally, all of Mr. Chadha’s outstanding and unvested equity awards vested in full as of September 1, 2023, and an exercise period of three years from that accelerated vesting date applied.
Director Compensation
The compensation program for our non-employee directors is intended to fairly compensate them for the time and effort required of a director. The Board takes into consideration the performance of the Company and the director’s role in committee assignments when determining the appropriate level of their compensation.
Director Cash Fees and Equity Awards
The current compensation arrangement for the non-employee directors of the Company, excluding Mr. Marchio, is as follows:
Cash Fees
•Annual cash fee of $44,000
•Additional annual cash fee of $20,000 for the Chairman of the Board
•Choice of an additional $10,000 in cash or 1,500 stock options
•Additional annual cash fee for Committee Chairs as follows:
•Audit Committee: $15,000
•Compensation Committee: $10,000
•Nominating and Corporate Governance Committee: $10,000
•Additional annual cash fee for non-Chair members of the Committees as follows:
•Audit Committee: $7,000
•Compensation Committee: $7,000
•Nominating and Corporate Governance Committee: $7,000
•All cash fees are payable on a quarterly basis
Equity Awards
•Annual Equity Awards
•65,000 stock options, granted on the date of the annual meeting of stockholders each year, beginning in 2022
•Vests 100% after one year of grant date
•New Board Members
•60,000 stock options granted on the date of appointment or election, as applicable, to the Board
•Vests 25% after one year of grant date, with the remainder on a monthly basis over the next three years
Non-employee directors are also reimbursed for any of their business expenses for each meeting attended.
Director Compensation Table
The table below summarizes the compensation paid by the Company to non-employee directors for the year ended December 31, 2023.
| | | | | | | | |
| Name | | Fees Earned or Paid in Cash
($) |
| Reginald Hardy | | 56,667 |
| Gary A. Lyons | | 51,000 |
| Vijay B. Samant | | 57,000 |
| Robert B. Brown | | 25,667 |
| Albert N. Marchio, II | | — |
Retention Agreements
We entered into employee retention bonus agreements, dated as of February 21, 2023, with Messrs. Sklawer, McAvoy and Chadha. Pursuant to the retention agreements, each executive officer was eligible to receive a cash bonus equal to 20% of his base salary in effect as of January 2, 2023, 50% of which (the “First Bonus”) was
earned if such executive officer remained employed by the Company through 11:59 p.m. MT on June 30, 2023 (the “First Bonus Eligibility Date”) and 50% of which (the “Second Bonus,” and together with the First Bonus, the “Bonuses”) was earned if such executive officer remained employed by the Company through 11:59 p.m. MT on December 31, 2023 (the “Second Bonus Eligibility Date”).
If an executive officer had been terminated without cause, died or become disabled (each, a “Specified Termination”) prior to the First Bonus Eligibility Date, he (or his estate) would have been entitled to receive only the First Bonus. Upon a Specified Termination after the First Bonus Eligibility Date, he (or his estate) was entitled to receive both Bonuses.
The retention agreements were subsequently amended to change the Second Bonus Eligibility Date to the Separation Date.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Securities
The following table sets forth information with respect to the beneficial ownership of our common stock as of March 13, 2024 by:
•our named executive officers;
•each of our directors;
•all of our executive officers and directors as a group; and
•each person or group of affiliated persons known by us to beneficially own more than 5% of our common stock.
The number of shares beneficially owned by each stockholder is determined under rules issued by the SEC. Under these rules, beneficial ownership includes any shares as to which a person has sole or shared voting power or investment power. Applicable percentage ownership is based on 5,973,306 shares of common stock outstanding as of March 13, 2024. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options or other rights held by such person that are currently exercisable or that will become exercisable or otherwise vest within 60 days of
March 13, 2024 are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Common Stock | | | Rights to Acquire Shares Within 60 Days of March 13, 2024(1) | | | Total Stock and
Stock-Based
Holdings | | | Percent of Total(2) | |
| 5% or Greater Stockholders | | | | | | | | | | | | |
Exploration Capital Fund, LP(3) Stephen L. Gustin 250 East 200 South, Floor 16 Salt Lake City, UT 84111 | | 1,010,842 | | | — | | | 1,010,842 | | | 16.9% | |
| Named Executive Officers and Directors |
| Albert N. Marchio, II | | — | | | — | | | — | | | * | |
Robert B. Brown(4) | | 7,034 | | | 3,257 | | | 10,291 | | | * | |
Andrew D. Sklawer(4) | | — | | | 357 | | | 357 | | | * | |
David R. McAvoy(4) | | 16,371 | | | 973 | | | 17,344 | | | * | |
Deepak Chadha(4) | | 22,314 | | | 728 | | | 23,042 | | | * | |
| All current directors and executive officers as a group (1 person) | | — | | | — | | | — | | | * | |
_________________
* Less than 1%
(1)Rights to acquire shares within 60 days of March 13, 2024 consist of warrants to purchase our common stock.
(2)Percent of shares beneficially owned by any person is calculated by dividing the number of shares beneficially owned by that person as of March 13, 2024 (including any shares which that person has the right to acquire beneficial ownership of within 60 days of March 13, 2024), by the sum of the total number of shares outstanding as of March 13, 2024, and the number of shares which that person has the right to acquire beneficial ownership of within 60 days of March 13, 2024. Applicable percentages are based on 5,973,306 shares of our common stock outstanding as of March 13, 2024.
(3)Based on a Schedule 13G filed with the SEC on January 17, 2024 and other Forms 3 and 4 filed with the SEC through March 13, 2024, Exploration Capital Fund, LP (the “Partnership”) is the beneficial owner of 927,404 shares of our common stock. Exploration Capital, LLC (“X-Cap”) is the investment manager of the Partnership and as a result may be deemed to be the beneficial owner of all the securities held by the Partnership. Exploration Capital General Partner, LLC (the “GP”) is the general partner of the Partnership and as a result may be deemed to be the beneficial owner of all the securities held by the Partnership. Stephen L. Gustin is the Managing Partner of X-Cap and as a result may be deemed to be the beneficial owner of all the securities held by the Partnership. Mr. Gustin disclaims beneficial ownership of the reported securities held by the Partnership except to the extent of his pecuniary interest. Mr. Gustin also has sole voting and dispositive power over 47,000 shares he directly owns.
(4)These named executive officers were no longer serving as executive officers as of March 13, 2024.
Equity Compensation Plan Information
The Company’s equity compensation plans were terminated in December 2023 in conjunction with the Company’s Plan of Dissolution. Therefore, as of December 31, 2023, the Company had no outstanding, issuable shares under any of the Company’s prior equity compensation plans.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Independence of the Board
Under the Nasdaq listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. Historically, our Board of Directors consulted with our General Counsel and external counsel to ensure that the Board’s determinations were consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of Nasdaq, as in effect from time to time.
Consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of their family members, and us, our senior management and our independent registered public accounting firm, our Board affirmatively determined that former directors Messrs. Lyons and Samant were independent directors within the meaning of the applicable Nasdaq listing standards. Currently, our Board has one member, Mr. Marchio, who is not independent.
On September 18, 2023, in connection with the approval of the Plan of Dissolution and the resignation of each of Messrs. Hardy, Lyons, and Samant from the Board, the Board dissolved the Company’s Audit, Compensation and Nominating and Corporate Governance Committees, effective October 2, 2023.
Certain Relationships and Related-Person Transactions
We have adopted a Related-Person Transactions Policy to monitor transactions in which the Company and any of the following have an interest: a director, executive officer, or other employee or a nominee to become a director of the Company; a security holder known by the Company to be the record or beneficial owner of more than 5% of any class of the Company’s voting securities; an “immediate family member” of any of the foregoing, which means any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law of such person, and any person (other than a tenant or employee) sharing the household of such person; and any firm, corporation or other entity in which any of the foregoing persons is an executive, partner or principal or holds a similar control position or in which such person directly or indirectly has a 5% or greater equity interest (collectively, “Related Persons”). The policy covers any transaction, arrangement, or relationship (or any series of similar transactions, arrangements, or relationships) in which the Company is, was, or will be a participant in which the amount involved exceeds $120,000 U.S. dollars and in which any Related Person had, has or will have a direct or indirect material interest (“Related-Person Transactions”). Transactions involving compensation for services provided to the Company as an employee, consultant, or director are not considered Related-Person Transactions under this policy.
Under this policy, any proposed transaction that has been identified as a Related Person Transaction may be consummated or materially amended only following approval by the Audit Committee or the Board in accordance with the provisions of this policy. In the event that it is inappropriate for the Audit Committee or the Board to review the transaction for reasons of conflict of interest or otherwise, after taking into account possible recusals by Audit Committee or Board members, then the Related Person Transaction shall be reviewed and decided upon by another independent member of the Board. Subsequent to the dissolution of the Compensation Committee effective October 2, 2023, the Board is responsible for reviewing and approving Related Person Transactions.
There were no Related-Person Transactions in 2022 or 2023.
Executive Compensation and Employment Arrangements
Information on compensation arrangements with the Company’s executive officers is described in detail in section Item 11. “Executive Compensation.”
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Fees of Principal Accounting Firm
The following table sets forth fees billed for professional audit services and other services rendered to the Company by Ernst & Young and its affiliates for the following fiscal years ended December 31:
| | | | | | | | | | | | | | |
| | Year Ended
December 31, |
| | | | |
| | 2023 | | 2022 |
| Audit Fees | | $ | 292,000 | | | $ | 473,750 | |
| Audit-Related Fees | | — | | — |
| Tax Fees | | — | | — |
| All Other Fees | | — | | — |
| Total | | $ | 292,000 | | $ | 473,750 |
Audit Fees. Audit fees consist of aggregate fees for professional services rendered for the audit of the Company’s annual consolidated financial statements and review of financial statements included in the Company’s Form 10-Q filings, and other services that are normally provided in connection with regulatory filings or engagements.
All fees described above were pre-approved by the Audit Committee of our Board.
PART IV.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements
The financial statements required by this item are submitted in a separate section beginning on page 35 of this Annual Report. (a)(2) Financial Statement Schedules
Financial statement schedules have been omitted because they are either not required, not applicable, or the information is otherwise included.
(a)(3) Exhibits
See Exhibit Index, which is incorporated herein by reference.
EXHIBIT INDEX
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Description of Exhibit | Form | Date of Filing | Exhibit Number | Filed Herewith | | | | | | | | | |
| 2.1 | | | 8-K | 9/19/2023 | 2.1 | | | | | | | | | | |
| 3.1 | | | 8-K | 9/8/2022 | 3.2 | | | | | | | | | | |
| 3.2 | | | 8-K | 9/19/2023 | 3.1 | | | | | | | | | | |
| 4.1 | | | S-8 | 9/10/2019 | 4.1 | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
4.2 | | | S-1 | 10/13/2020 | 4.2 | | | | | | | | | | |
4.3 | | | S-1 | 10/13/2020 | 4.4 | | | | | | | | | | |
4.4 | | | S-1/A | 6/17/2020 | 4.4 | | | | | | | | | | |
4.5 | | | S-1/A | 6/17/2020 | 4.2 | | | | | | | | | | |
4.6 | | | 10-K | 3/30/2023 | 4.80 | | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | |
| 10.1† | | | 8-K | 9/1/2021 | 10.1 | | | | | | | | | | |
| 10.2† | | | 8-K | 2/2/2022 | 10.1 | | | | | | | | | | |
10.3† | | | 8-K | 3/7/2024 | 10.1 | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
10.4† | | | 8-K | 5/3/2022 | 10.1 | | | | | | | | | | |
10.5† | | | 10-Q | 11/14/2022 | 10.6 | | | | | | | | | | |
10.6† | | | 8-K | 5/3/2022 | 10.2 | | | | | | | | | | |
10.7 | | | 8-K | 7/21/2023 | 10.1 | | | | | | | | | | |
10.8† | | | 8-K | 9/3/2019 | 10.2 | | | | | | | | | | |
10.9† | | | S-1/A | 6/8/2020 | 10.2 | | | | | | | | | | |
10.10† | | Amendment No. 2 to License, Development and Commercialization Agreement, dated October 6, 2017, by and between Brickell Biotech, Inc. and Kaken Pharmaceutical Co., Ltd., including Right of First Negotiation Agreement, as amended, dated October 6, 2017, by and between Brickell Biotech, Inc. and Kaken Pharmaceutical Co., Ltd. | 8-K | 9/3/2019 | 10.3 | | | | | | | | | | |
10.11† | | | S-1/A | 6/8/2020 | 10.4 | | | | | | | | | | |
10.12† | | | S-1/A | 6/8/2020 | 10.5 | | | | | | | | | | |
10.13† | | | S-1 | 10/13/2020 | 10.6 | | | | | | | | | | |
10.14† | | | 10-K | 3/9/2021 | 10.7 | | | | | | | | | | |
10.15† | | | 10-Q | 8/12/2021 | 10.4 | | | | | | | | | | |
10.16† | | | 8-K | 2/18/2020 | 10.1 | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
10.17† | | | 8-K | 2/18/2020 | 10.2 | | | | | | | | | | |
10.18† | | | 8-K | 5/3/2022 | 10.3 | | | | | | | | | | |
10.19† | | | 10-Q | 11/14/2022 | 10.7 | | | | | | | | | | |
10.20 | | | 8-K | 7/21/2023 | 10.2 | | | | | | | | | | |
| | | | | | | | | |
| 10.21 | | | 8-K | 9/3/2019 | 10.10 | | | | | | | | | | |
10.22 | | | 10-Q | 8/12/2021 | 10.1 | | | | | | | | | | |
10.23 | | | 10-K | 3/30/2023 | 10.20 | | | | | | | | | | |
10.24 | | | 10-Q | 5/10/2023 | 10.9 | | | | | | | | | | |
| | | | | | | | | |
10.25+ | | | 10-K | 3/30/2023 | 10.21 | | | | | | | | | | |
10.26+ | | | 8-K | 2/7/2023 | 10.1 | | | | | | | | | | |
10.27 | | | 8-K | 1/27/2023 | 10.1 | | | | | | | | | | |
10.28+ | | | 8-K | 2/24/2023 | 10.1 | | | | | | | | | | |
10.29+ | | | 8-K | 10/10/2023 | 10.1 | | | | | | | | | | |
10.30 | | | 8-K | 10/10/2023 | 10.2 | | | | | | | | | | |
10.31+ | | | 8-K | 11/24/2020 | 10.2 | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
10.32+ | | | 8-K | 2/24/2023 | 10.2 | | | | | | | | | | |
10.33+ | | | 10-Q | 11/13/2023 | 10.7 | | | | | | | | | | |
10.34+ | | | 10-K | 3/30/2023 | 10.27 | | | | | | | | | | |
10.35+ | | | 10-Q | 11/13/2023 | 10.5 | | | | | | | | | | |
10.36 | | | 10-Q | 11/13/2023 | 10.6 | | | | | | | | | | |
10.37+ | | | 10-K/A | 5/1/2023 | 10.29 | | | | | | | | | | |
10.38+ | | | 8-K | 2/24/2023 | 10.3 | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| 21.1 | | | | | | × | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| 31.1 | | | | | | × | | | | | | | | | |
| | | | | | | | | | | | | | | |
| 32.1* | | | | | | × | | | | | | | | | |
| 101.INS | | Inline XBRL Instance Document | | | | × | | | | | | | | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document | | | | × | | | | | | | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | × | | | | | | | | | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document | | | | × | | | | | | | | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document | | | | × | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | | | × | | | | | | | | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | | | | × | | | | | | | | | |
__________________
| | | | | |
| + | Indicates a management contract or compensatory plan. |
| |
| † | Certain confidential information contained in this agreement has been omitted because it is both not material and is the type that the registrant treats as private or confidential. |
| * | This certification is being furnished pursuant to 18 U.S.C. Section 1350 and is not being filed for purposes of Section 18 of the Exchange Act and is not to be incorporated by reference into any filing of the Registrant, whether made before or after the date hereof. |
| |
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | | | |
| | Fresh Tracks Therapeutics, Inc. | | |
| | | | | | |
| Date: March 15, 2024 | | By: | | /s/ Albert N. Marchio, II | | |
| | | | Albert N. Marchio, II Chief Executive Officer and Chief Financial Officer (Principal Executive Officer and Principal Financial Officer) | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | | | |
| /s/ Albert N. Marchio, II | | Chairman of the Board Chief Executive Officer and Chief Financial Officer (Principal Executive Officer and Principal Financial Officer) | | March 15, 2024 |
| Albert N. Marchio, II | | |
| | | | |
| /s/ Aaron Fox-Collis | | VP of Finance and Chief Accounting Officer
(Principal Accounting Officer) | | March 15, 2024 |
| Aaron Fox-Collis | | |
| | | | |